Research Article - (2025) Volume 11, Issue 2
Received: 29-May-2024, Manuscript No. JPNP-24-137565;
Editor assigned: 03-Jun-2024, Pre QC No. JPNP-24-137565 (PQ);
Reviewed: 18-Jun-2024, QC No. JPNP-24-137565;
Revised: 29-Mar-2025, Manuscript No. JPNP-24-137565 (R);
Published:
06-Apr-2025
, DOI: 10.37421/2472-0992.2025.11.351
Citation: Bushra Suliman, Bushra Elfatih. "Phytochemical Analysis and Antimicrobial Investigation of Root Bark Extracts of La'ot (Acacia nubica Benth.) from Sudan." J Pharmacogn Nat Prod 11 (2025): 351.
Copyright: © 2024 Suliman BEB. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Plant compounds are of interest as a source of safe and effective substitutes of the classic synthetically produced antimicrobial agents. Hence, this study aims to investigate the phytochemical constituents and antimicrobial activity present in Acacia nubica root bark extracts. The plant was extracted by maceration using ethanol (70%). The crude extract was used for the detection of the phytochemical constituents. The antibacterial activity was measured by the agar well diffusion method. The qualitative chemical analysis of the crude organic extracts confirmed the presence of diterpenes, cardiac glycosides, tannins, flavonoids, saponins and phenols. The chemical ingredients were identified by Gas Chromatography-Mass Spectrometry (GC-MS). The major constituents in crude ethanol extract were 5-hydroxymethylfurfural (3.96%), di-n-octyl phthalate (4.33%), n-hexadecanoic acid (1.6%), and 4-O-methylmannose (81.11%). While the major constituents of aqueous methanol fraction were 4-O-methylmannose (74.98%), 5 hydroxymethylfurfural (12.45%), and 3-buten-2-one, 4-(4-hydroxy-3-methoxyphenyl) (3.74%), and of the hexane fraction were Di-n-octyl phthalate (54.92%), hexanedioic acid, bis (2-ethylhexyl) ester (9.9%), and hexadecanoic acid, ethyl ester (8.18%). A. nubica crude ethanolic extract produced clear zone of inhibition (9.00-12.5 mm) in the antimicrobial assay. The aqueous methanol fraction produced clear zone of inhibition of 11.00-13 mm against Staphylococcus aureus (ATCC 25923), while the hexane fraction was found to be devoid of activity. Escherichia coli (ATCC 25927) proved to be resistant to the crude extract and its fractions. On the other hand, ethanol (70%) extract showed antifungal activity with minimal inhibitory effect of 12.2-13.2 mm against C. albicans clinical isolates, while the aqueous methanol fraction produced 13.3–14.43 mm clear zone of inhibition. The hexane fraction produced no inhibition of the fungal growth. Further, studies on phytochemical, antimicrobial and mechanism of action are needed.
Antimicrobial agents • Acacia nubica •Ethanol • Gas chromatography-mass spectrometry • Staphylococcus aureus • Escherichia coli • C. albicans
The plant compounds are of interest as a source of safe and effective alternatives than the classic synthetically produced antimicrobial agents. Veing reliable and accurate methods of screening plants constituents, phytochemical analysis received great attention in the last years leading to a great progress in methods and technologies. With the aid of these procedures numerous plants-derived components with benefits to the medical field have been identified [1].
The pharmacologically active components in medicinal plants are known as active principles. The active principles are divided chemically into several groups among which are: alkaloids, volatile essential oils, phenols and phenolic glycosides, resins, oleosins, steroids, tannins and terpenes [2].
Sudan is endowed with many plants that are potential sources of drugs to treat diseases. Medicinal use of plants, as traditional herbal remedies, is common and of importance in Sudan. One of the plants that are traditionally used for medicinal purposes in Sudan is Acacia nubica [3].
Acacia genus: Acacia genus is a large genus about 1350 species in the family Fabaceae. These species are considered substantial sources of gallic and ellagic acids [4]. Most of these species contain flavonoids beside other phenolics [5]. In ethnomedicine some Acacia species are used as antidiabetic, hypotensive, antiamoebic, antidiarrheal and anti-inflammatory [6]. The antimicrobial potential of many Acacia species has been documented [7]. In Sudanese folk medicine Acacia nilotica is used against cough, sore throat, malaria, intestinal worms and wounds [8]. Pods of A. nilotica are used commercially in Sudan for leather tanning [9]. The effect of the aqueous extract of A. nilotica pods on diabetic animal models has been studied. Diabetic rats exhibited hypoglycemia, significant increase in lipid peroxidation and elevated serum urea and creatinine [10]. The gum from A. seyal Gum Arabic finds many traditional uses including kidney disorders. Though the pharmacological effects of Gum Arabic were extensively investigated in animal models, there is paucity of data regarding quantified use in humans [11]. Acacia gum has been used as demulcent in pharmaceutical preparations. The gum has been used traditionally for healing wounds and has been shown to inhibit early deposition of plaque. The antioxidant capacity of the medicinally important species A. auricoliformis has been evaluated [12]. The ethanol extract of A. aroma showed significant activity against gram positive bacteria [13].
Antibiotic resistance
Antibiotics are one of the necessary components in treating diseases originating from bacterial infections. Antibiotics themselves are usually created from microorganisms that are toxic to other microorganisms (bacteria). Despite the effectiveness of antibiotics in treating diseases caused by bacterial infections, their extensive use has resulted in antibiotic resistance. Plants were known to produce compounds that inhibit bacterial growth (bacteriostatic). The natural antibiotic approach is expected to reduce the problem of antibiotic resistance. Natural antibiotics also have some advantages both for the user and the environment. The use of herbal ingredients has fewer side effects. In particular, the herbal component has a multifunction ability to treat several diseases at once. On top of that, herbal ingredients are better for the surrounding environment because they require less industrial processing and necessitate growing more plants [14].
Meanwhile during a time of rapidly rising antibiotic resistance, new approaches are necessary to enrich the antimicrobial drug development pipeline. Moving forward, there are clearly several innovative strategies to pursue in the search for novel therapies. Plants remain a unique and underexploited source of bioactive compounds, and ethnobotanical research tools can be used to guide future research efforts and narrow down the search to the most likely source candidates. In addition to tests for classic bacteriostatic and bactericidal activity, it is also essential to examine complex plant extracts and individual compounds for activity against alternative bacterial targets [15].
Problem identification and justification
Recently antimicrobial resistance and infectious diseases have increased which raises concerns about what will be the next step in facing bacterial and fungal infections. Knowing that there are millions of undiscovered compounds inside the plants, some of them possess strong antimicrobial activity and moreover it is easy to manufacture a dosage form of plant origin for patient use and safer than most of the synthetic antimicrobial agents.
The intersection of diverse cultures and the unique geography holds great potential for Sudanese herbal medicine. This study aims to shed the light upon A. nubica being one of the most widely distributed plants at Central Sudan aiming to establish the scientific base of its medicinal and phytochemical properties.
Objectives
General objective: To Investigate the phytochemical compounds and antimicrobial activity of A. nubica root bark extracts.
Specific objectives
• To extract the A. nubica plant sample by maceration using ethanol (70%).
• To perform liquid-liquid fractionation of alcoholic extract to obtain polar and non-polar fractions.
• To determine, qualitatively and quantitatively, the chemical constituents present in A. nubica root bark extracts by chemical test and GC-MS and fraction analysis.
• To preform antibacterial activity test of A. nubica root bark extract against standard bacterial strains: E. coli (ATCC 25927) and S. aureus (ATCC 25923)
• To perform antifungal activity test of A. nubica root bark extract against C. albicans clinical isolate.
Acacia genus: Acacia genus is a large genus, of the family Fabaceae [16]. About 1350 Acacia species are widely dispersed in the Americas, the Carribbean and Pacific Islands, Africa, Madagascar and the Mascarenes, Asia, the Indo-Malesian region and Australia. Europe is the only large geographical area devoid of indigenous Acacia species, and there are no indigenous species in New Zealand despite its relative proximity to Australia. The fossil record indicates that the genus was previously more widely distributed having been present formerly in the Ukraine and in New Zealand. Most species of Acacia occur in regions where the rainfall is markedly seasonal or low, relatively few inhabiting rainforest areas, but even then, the rainfall is usually unevenly distributed throughout the year and even in the wettest parts there is usually a short dry season. This does not imply that the genus originated in an arid or semi-arid region. On the contrary, it is considered probable that Acacia originated in the tropical lowlands and that most of the xerophytic features within the genus are secondary [17].
Acacia species are substantial sources of gallic and ellagic acids [18]. Most of these species contain flavonoids beside other phenolics [19]. In ethno medicine some Acacia species are used as ant diabetic, hypotensive, antiamoebic, antidiarrheal and anti-inflammatory [20]. The antimicrobial potential of many Acacia species has been documented [21]. In Sudanese folk medicine A. nilotica is used against cough, sore throat, malaria, intestinal worms, and wounds [22]. Pods of A. nilotica are used commercially in Sudan for leather tanning [23]. The effect of the aqueous extract of A. nilotica pods on diabetic models has been studied. Diabetic rats exhibited hypoglycemia, significant increase in lipid peroxidation and elevated serum urea and creatinine [24]. The gum from A. seyal-Gum Arabic finds many traditional uses including kidney disorders. Though the pharmacological effects of Gum Arabic were extensively investigated in animal models, there is paucity of data regarding quantified use in humans [25]. Acacia gum has been used as demulcent in pharmaceutical preparations. The gum has been used traditionally for healing wounds and has been shown to inhibit early deposition of plaque. The antioxidant capacity of the medicinally important species A. auricoliformis has been evaluated [26]. The ethanol extract of A. aroma showed significant activity against gram positive bacteria [27].
Acacia nubica: A. nubica Benth. (A. oerfota (Forssk.) Schweinf.) Leguminosae family, known in Sudan as Laout or El Ifein, a greengrey or whitish-green multi-stemmed shrub 1-5 m high with basal branching merged into an irregular or obconical crown. It is distributed through the arid and semi-arid zones or short grass savanna woodlands of Africa to Arabia, Persia, and India in Asia. In Sudan, known as La’ot, Laout or El Ifein, A. nubica usually occupied dry-hard, bared, and exhausted clay fields and rocky-dry slopes under 100-200 mm rainfall of Central and Northern parts. It is capable to germinate at temperatures ranging from 20 up to 40°C. Due to withstand of extremely harsh conditions Alshafia et al., A. nubica is an indispensable component of agropastoral systems in dry areas and with exceedingly fundamental environmental roles. Rural peoples within the arid area where A. nubica grows rely on the plant to provide their daily needs of medicines, gums, fuel, fiber, timber, fodder, veterinary practices, and apiculture system. Analysis of the plant parts showed high contents of crude protein, essential minerals, nitrogen degradation and dry matter digestibility with relatively low tannins contents. In addition, it helps in improving soil fertility through nitrogen fixation and leaf litters as well as stabilizing soils against land degradation and desertification. The miscellaneous services delivered by A. nubica and its remarkable adaptability to harsh environments and growth ability in degraded lands make it critical in the semi-arid and savannah regions. However, in Sudan, due to human activities and climate change the natural habitats of A. nubica severely deteriorated in a way that, A. nubica cover was decreased by 9.72% in North Kordofan, and stated to became infrequent in Gezira state [28].
Botanical classification of A. nubica:
Kingdom: plantae
Division: Magnoliophyt
Class: Magnoliopsida
Order: Fabales
Family: Fabaceae
Sub family: Mimosoideae
Genus: Acacia
Species: A. oerfota
Synonyms:
• A. virchowiana Vatke
• A. merkeri Harms.
• A. oerfota.
• A. nubica
Common names La’ot (Sudan).
Geographical distribution: A. nubica is a multipurpose tree with extensive geographic distribution from Egypt to Mauritania and South Africa in Africa and from East Asia to India, Pakistan, and Iran in Asia. In Sudan, the plant natural habitat is mainly in Gazira state, Kordofan and Blue Nile state [29].
Botanical description: A. nubica is a dicot, small shrub with flatted top, triangular funnel in vertical, branches numerous and grey; spines straight, long, hard, white with brown tip, grouped in pairs. Leaves are long and flowers are whitish, very fragrant, and clustered in balls (Figure 1). Pods are flat and elongated, while seeds are of olive-green color [30].
Figure 1. A. nubica in Sudan.
Phytoconstituents: A. nubica is a pioneer species, relatively high in bioactive secondary compounds and it is important for a variety of functions. The plant is economically used as a source of tannins, gums, timber, fuel and fodder A. nubica is a pantropical and subtropical genus with species abundant throughout Asia, Australia, Africa and America. A. nubica grows naturally and it is imperative in traditional rural and agro-pastoral systems. A. nubica is an imperative multipurpose plant that has been used broadly for the treatment of various diseases. In other studies, it has been reported that Acacia species contains secondary metabolites including amines and alkaloids, cyanogenic glycosides, cyclitols, fatty acids and seed oils, fluoroacetate, gums, nonprotein amino acids, terpenes (including essential oils, diterpenes, phytosterol and triterpene genins and saponins), hydrolysable tannins, flavonoids and condensed tannins. The plant is a rich source of cysteine, methionine, threonine, lysine, tryptophan, potassium, phosphorus, magnesium, iron and manganese [31].
Natural products as source of antimicrobial agents: Nature has served as humankind’s pharmacy for millions of years. Indeed, selfmedication with natural resources such as plants and fungi has not been restricted to human use only, but has even been documented in various animals, ranging from insects to primates. Plants produce complex suites of compounds known as secondary metabolites, which are not necessary for their primary growth and function, but rather serve another role of enhancing likelihood of survival. Plants are sessile and thus highly dependent on the ability to produce and release these chemical signals into their environment for the purposes of communication and defense. Throughout ancient history, humans have learned to harness this chemical arsenal to serve their own needs. This is most apparent when considering human health and traditional forms of medicine [32].
The development of antimicrobial products from plant extracts is incredibly chemically complex compared to development from other sources. fungi as a single extract preparation may contain hundreds of different chemical entities. The isolation of single compounds with the desired antimicrobial bioactivity can be time-consuming and requires a large amount of bulk plant material. Making arrangements for access to plant specimens can sometimes be difficult, especially in an international setting. Regulations concerning plant collection permits and export/import permits, which differ depending on where the research is being conducted [33]. Furthermore, as per the regulations and guidance set forth by the United Nations Convention on Biological Diversity and the Nagoya Protocol, negotiation of equitable access and benefit sharing agreements is required for such research. Many plantbased therapies work via synergistic pathways. Synergism among compounds in a complex mixture presents unique difficulties as the scientific technology to study multiple compounds acting in unison on potentially multiple biological targets has not yet been fully developed. On the other hand, it could be argued that the synergistic activity of certain plant extracts may present a unique opportunity in the face of growing antibiotic resistance. It raises the question of whether more chemically complex formulations can outlast monotherapies by making it more difficult for microbes to evolve resistance to a multi-sided attack [34].
Antimicrobial Activity of A. nubica: A. nubica arial parts extracts, ethanol, chloroform and acetone exhibited antimicrobial activity against two standard bacterial strains of Gram +ve bacteria (S. aureus: ATCC 25923), Gram −ve bacteria (P. aeruginosa: ATCC 27853) and standard fungi Candida albicans (ATCC 90028) using the agar-plate well diffusion method. The chloroform extract was inactive compared to ethanol and acetone extracts. But ethanol extract showed the maximum antimicrobial activity against the tested organisms. Amongst the plant species screened, ethanol extract of A. nubica arial parts showed maximum inhibitory activity (31 mm), (22 mm) and (27) against S. aureus, P. aeruginosa and C. albicans, respectively [35].
Several studies documented the activity of different parts of A. nubica against microbial agents, when tested against Madurella mycetomatis, methanol extract of A. nubica root bark inhibited M. mycetomatis growth at a concentration as low as 1 μg/ml [36].
Materials
Chemicals and reagents: The chemicals with the company and source show in Table 1.
|
Chemicals |
Company |
Source |
|
Methanol |
(S.D. Fine Chem Limited) |
India |
|
Hexane |
(S.D. Fine Chem Limited) |
India |
|
Ceftriaxone |
(Himedia Ref-1pk) |
India |
|
Vancomycin |
(Himedia Ref-1pk) |
India |
|
Muller Hinton’s agar |
(Himedia Ref-1pk) |
India |
Table 1 . List of chemicals with the company and source.
Glassware and instruments: Beakers, conical flask, round bottom flak, petri dishes, cylinders, test tube, etc., were obtained from (SanaiLab BORO 3.3 KSA). Electrical blinder (Mounlinex blender the genuine 400 W, France). Gas Chromatography-Mass Spectrometry (GC/MS-QP2010SE, Shimadzu, Japan serial number: 020535400496SA).
Tested microbial strains: The standard bacterial strains for the antimicrobial activity assay (S. aureus (ATCC 25923) and E. coli (ATCC 25927)) were obtained from faculty of medical laboratory sciences, university of Gezira.
The fungal strain for the antimicrobial activity assay (C. albicans clinical isolate) was obtained from faculty of medical laboratory sciences, university of Gezira.
Methods
Sample collection and authentication: The plant material was collected from Gezira state (Central Sudan) during summer season (July 2022). The plant species was identified at the herbarium of the department of pharmacognosy, faculty of pharmacy, university of Gezira, Wad Medani, Sudan.
A. nubica roots were authenticated at medicinal and aromatic plants research center faculty of pharmacy, university of Gezira, Wad Medani, Sudan by Prof. Elhadi Mohamed Mohamed Ahmed.
Processing of plant sample: The roots barks were peeled using hand and seizer, cleaned from dust and foreign matters, and cut into small pieces using seizer then milled to coarse powder with the help of mortar and pestle then electrical blender.
Extraction of plant sample: The dried powdered root bark (100 g) was extracted by maceration using 1 L ethanol 70%, filtered using Buchner funnel and concentrated by open air evaporation for 3 days until to dryness, the yield was then stored in refrigerator.
Liquid-liquid fractionation: In this method 2 grams of the extract collected from the maceration was fractionated using aqueous methanol (70%) and hexane in separatory funnel to produce two fractions (aqueous fraction, hexane fraction). Both fractions were evaporated in open air to collect dried sample of each fraction.
GC-MS analysis: GC-MS analysis was done for the two fractions and the crude using a GC-MS instrument with the following setup: Injector temperature 300°C, in split mode. Oven temperature was (60°C-300°C) at 10°C/min. The carrier gas was helium at flow rate (1.6 ml/min), Volume of injection was 1 μL. Ion source of mass spectroscopy temperature was 200°C and the interface temperature was 250°C, the mass scan range (m/z) was 40-500 m/z. The spectrum of the components was compared with the database of spectrum of known components stored in the GC-MS library (NIST).
Phytochemical screening methods: The Crude extract was used for the following tests according to standard methods described by Abdllha et al.
Test for phenols: Two ml of extract were added to one ml of distilled water and warmed at 45°C-50°C, then 2 ml of 3% ferric chloride was added. Appearance of green or blue color indicated the presence of phenols.
Test for flavonoids : One ml of extract was added to one ml of 10% KOH. It was gently shaken. Appearance of yellow color indicated the presence of flavonoids.
Test for tannins: One ml of the extract was added to one ml of 3% ferric chloride. A greenish precipitate indicated the presence of tannins.
Test for alkaloids: One ml of Dragendorff reagent was added to one ml of filtrate. The formation of cloudy orange indicated the presence of alkaloids.
Test for terpenoids and steroids: Five ml of extract was mixed in two ml of chloroform. Then three ml concentrated sulphuric acid was added carefully to observe for a reddish-brown coloration between the two layers that indicates the presence of terpenoids and steroids.
Test for saponins: Approximately 0.2 ml of extract was mixed with 5 ml of distilled water, mixture was shaken vigorously for 5 min. Persistence of foams indicated the presence of saponins.
Test for glycosides (Keller Killiani test): 0.5 g of extract was added to 0.4 ml glacial acetic acid containing a trace amount of ferric chloride solution and then a 0.5 ml of concentrated sulphuric acid was gently added along the sides of the test tube. Appearance of a blue color in the acetic acid layer indicated the presence of cardiac glycosides [37].
Test for amino acids (Ninhydrin test): To the extract, 0.25% w/v ninhydrin reagent was added and boiled for 5 min. Formation of a blue color indicated the presence of amino acids.
Test for carbohydrates (Molish’s test): The extract was treated with a few drops of alcoholic α-naphthol. Then 0.2 ml of concentrated sulphuric acid was slowly added along the sides of the test tube. Appearance of a purple to violet color ring at the junction indicates the presence of carbohydrates.
Biological testing preparations
Preparation of bacterial inoculum suspensions: The stock cultures of microorganisms used in this study were maintained on plate count agar slants at 4°C. Inoculum was prepared by suspending a loop full of bacterial cultures into 10 ml of nutrient agar broth and was incubated at 37°C for 24 hrs. Using wire loop each bacterium was washed-off with sterile normal saline to produce a suspension equivalent to of 0.5 McFarland’s standards (approximately 1 × 10 s Colony Forming Units (CFU)/ml). This suspension was used for streaking the agar plate with sterile swap.
Preparation of fungal inoculum suspension: The stock cultures of microorganisms used in this study were maintained on plate count agar slants at 4°C. Inoculum was prepared by suspending a loop full of bacterial cultures into 10 ml of Sabouraud dextrose agar broth and were incubated at 35°C for 24 hrs. Using wire loop the inoculum was washed-off with sterile normal saline to produce a suspension equivalent to of 0.5 McFarland’s standards (approximately 1 × 10 s Colony Forming Units (CFU)/ml). This suspension was used for streaking the agar plate with sterile swap.
In vitro antimicrobial activity tests
Antibacterial activity test: The cup-plate agar diffusion method was adopted with some minor modifications to assess the antibacterial activity of the prepared extracts. 100 ml of sterile molten nutrient agar were maintained at 45°C then, 20 ml aliquots of the inoculated nutrient agar were distributed into sterile Petri-dishes. The agar was left to dry and in each of these plates with the help of sterile cotton swap the standardized bacterial stock suspension 108-109 C.F.U/ml was streaked on the surface of each plate then 3 cups (8 mm in diameter) was cut using the back of a sterile blue tip and the agar discs were removed. Alternate wells were filled with 0.1 ml of each sample and the third well was filled with 0.1 solvent as negative control using automatic microliter-pipette and allowed to diffuse at room temperature for two hours, then the positive control antibiotic discs were placed using sterile forceps. The plates were then incubated in the upright position at 37°C for 18 hrs. Two replicates were carried out for each sample against each of the test organisms. After incubation, the diameters of the resultants and growth inhibition zones were measured and the mean values were documented.
Antifungal activity test: The inoculum used was prepared using the yeasts from a 24 hrs culture on Sabouraud dextrose agar. A suspension was made in a sterile saline solution (0.85%). The turbidity of the suspension was adjusted to obtain a final concentration to match that of a 0.5 McFarland standard (0.5-2.5 × 103). 20 ml of Sabouraud dextrose agar were melted, cooled to 55°C and then inoculated with 1 ml of the organism suspension. The inoculated agar was poured into the assay plate and allowed to cool down on a leveled surface. Once the medium had solidified, three wells, 3 cups (8 mm in diameter) were cut using the back of a sterile blue tip and the agar discs were removed. Alternate wells were filled with 0.1 ml of each sample and the third well was filled with 0.1 solvent as negative control using automatic Microliter-pipette and allowed to diffuse at room temperature for two hours the positive, then control antibiotic discs were placed using sterile forceps. The plates were then incubated in the upright position at 35°C for 18 hrs. Two replicates were carried out for each sample against each of the test organisms. After incubation, the diameters of the resultants and growth inhibition zones were measured and the mean values were documented.
Yield percentages of A. nubica root bark extract and its fractions
The result of extract and fractions yield of A. nubica root’s bark extract show in Table 2.
| Extract | Percentage | Color |
| Crude extract | 18.70% | Dark brown |
| Hexane fraction | 15.30% | Yellow color |
| Methanol fraction | 84.70% | Brown color |
Table 2. Showing the result of extract and fractions yield of A. nubica root’s bark extract.
Qualitative phytochemical analysis
Using standard procedures for preliminary phytochemical analysis, A. nubica crude extract was positive for the presence of flavonoids, saponins, phenols, tannins and terpenoids (Table 3), while alkaloids where not found.
| Test | Crude extract |
| Diterpenes | ++ |
| Cardiac glycosides | ++ |
| Tannins | + |
| Flavonoids | ++ |
| Saponins | + |
| Alkaloids | - |
| Phenols | +++ |
| Note: *(-) Indicates negative results, (+) slightly positive, (++) positive, (+++) highly positive. | |
Table 3. Preliminary phytochemical analysis of A. nubica root bark extract.
GC-MS analysis
GC-MS profiling of the extracts revealed the presence of compounds belonging to different chemical classes and most of them are known to exhibit important biological activities. Figures 2-4 showed the distinct chromatograms of A. nubica root bark extracted in different solvents. The major identified compounds with their peaks, structures, Retention Times (RT), and peak areas (%) are presented in Tables 4-6.
Figure 2. GC-MS based chemical profiling of A. nubica root bark ethanol 70% extract.
| Peak No. | Compound name | Structure | Retention time | Area% | |
| 1 | 5-Hydroxymethylfurfural |
|
14.424 | 3.96 | |
| 2 | 4-O-Methylmannose |
|
27.189 | 81.11 | |
| 3 | n-Hexadecanoic acid |
|
28.998 | 1.85 | |
| 4 | Di-n-octyl phthalate |
|
36.681 | 4.33 |
Table 4. Major phytochemical components identified in ethanol 70% crude extract of A. nubica by GC-MS.
Figure 3. GC-MS based chemical profiling of A. nubica root bark methanol fraction.
| Peak No. | Compound name | Structure | Retention time | Area% | |
| 1 | 5-Hydroxymethylfurfural |
|
14.65 | 12.45 | |
| 2 | 4-O-Methylmannose |
|
28.341 | 74.98 | |
| 3 | 3-Buten-2-one, 4-(4-hydroxy-3-methoxyphen |
|
32.065 | 3.74 | |
| 4 | Di-n-octyl phthalate |
|
36.681 | 0.49 |
Table 5. Major phytochemical components identified in methanol fraction of A. nubica by GC-MS.
Figure 4. GC-MS based chemical profiling of A. nubica root bark hexane fraction.
| Peak No. | Compound name | Structure | Retention time | Area% | |
| 1 | Hexadecanoic acid, ethyl ester |
|
29.425 | 8.18 | |
| 2 | Hexanedioic acid, bis(2-ethylhexyl) ester |
|
34.85 | 9.99 | |
| 3 | Di-n-octyl phthalate | 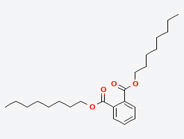 |
36.785 | 54.92 |
Table 6. Major phytochemical components identified in hexane fraction of A. nubica by GC-MS.
Antimicrobial activity of A. nubica root bark extracts
Three root bark extracts (hexane fraction, aqueous methanol fraction, and (70%) ethanol) of A. nubica were subjected to antibacterial assays against S. aureus (ATCC 25923) and E. coli (ATCC 25927). The mean Zones of Inhibition (mZOI) were measured as shown in (Figure 5). Both crude ethanol (70%) and aqueous methanol fraction of A. nubica root bark exhibited antibacterial activity against S. aureus (ATCC 25923) based on mZOIs. While hexane fraction was devoid of activity. A. nubica crude ethanolic extract displayed clear zone of inhibition of 9.00–12.5 mm and aqueous methanol fraction produced 11.00–13 mm clear zone of inhibition against S. aureus compared to vancomycin (13 mm). On the other hand, ethanol (70%) showed antifungal activity with minimal inhibitory effect of 12.2-13.2 mm, while aqueous methanol fraction produced 13.3–14.43 mm zone of inhibition against C. albicans (Figure 6). E. coli (ATCC 25927) was found to be resistant to all study extracts.
Figure 5. The susceptibility of S. aureus (ATCC 25923) to A. nubica root bark extracts at different concentrations compared to Vancomycin, mZOIs: mean Zone of growth Inhibitions.
Figure 6. The susceptibility of C. albicans clinical isolate to A. nubica root bark extracts at different concentrations compared to Amphotericin B, mZOIs: mean Zone of growth Inhibitions.
The yield percentage of alcoholic crude extract was (18.7%) of dark brown color with characteristic aroma extract. After liquid-liquid extraction, the yield from 2 grams of the crude extract were (15.3%) of hexane fraction with characteristic yellow color and (84.7%) of aqueous methanol fraction with characteristic brown color.
This study revealed the existence of a significant difference in the extract yield obtained by different solvents. Further, the highest extract yields and total phenolic content obtained in the aqueous methanolic Root bark fraction of A. nubica could be attributed to the polarity and nature of constituents therein. It was reported that the level of solubility of a compounds decides the recovery percentage of a particular solvent type.
Secondary metabolites flavonoids, saponins, phenols, tannins and terpenoids may be responsible, through different mechanisms, for the reported antimicrobial activity of the plant extracts. All extracts of A. nubica showed negative tests for alkaloids. Both fractions of A. nubica showed presence of the same set of secondary metabolites tested in this study (Table 1).
However, it was observed that aqueous fraction when compared to hexane fraction have potentially higher concentration of diterpenes, and tannins as shown by more intense color formations, this support that the activity was observed in ethanol 70% and aqueous methanol fraction, thus diterpenes and tannins could potentially account for the significantly higher antibacterial activity observed in A. nubica extract this result and findings agrees with the work done by Abdoelftah et al.
GC-MS chromatogram of the aqueous methanol extract showed 24 identified compounds with 3 major peaks. The major compounds included 4-O-Methylmannose (74.98%), 5-Hydroxymethylfurfural (12.45%), 3-Buten-2-one, 4-(4-hydroxy-3-methoxyphenyl) (3.74%). In the GC-MS profile of hexane fraction, 26 compounds were detected. The major compounds identified were Di-n-octyl phthalate (54.92%), Hexanedioic acid, bis(2-ethylhexyl) ester (9.9%), Hexadecanoic acid, ethyl ester (8.18%). While crude ethanol (70%) crude extract profile revealed the presence of 22 detectable compounds, including 5-Hydroxymethylfurfural, Di-n-octyl phthalate, n-Hexadecanoic acid, and 4-O-Methylmannose, as major components. Several studies documented that methoxy mannose contribute to the antimicrobial activity of different plants extracts specially at high concentrations as determined by molecular docking.
Considering the antimicrobial activity, A. nubica root bark extracts showed dose dependent antimicrobial activity against S. aureus and C. albicans this could be attributed to the fact that Gram positive bacteria generally lack the outer membrane, exposing them to antibiotics. Without the outer membrane, secondary metabolites can penetrate the peptidoglycan envelope and reach the cell membrane of gram-positive bacteria easier.
Conversely, gram-negative bacterial cell walls outer membrane (a lipopolysaccharide) is thought to act as a barrier to many substances including antibiotics. This was clearly observed in this study whereas, gram-negative bacteria, E. coli conferred resistance to the extracts.
Studies suggest that Acacia species possess secondary metabolites that were found to exhibit similar results to those obtained in this study with varying degree in potency. The differences in potency may be due to geographical sources of the plant species, time of collection of the plant sample, storage condition, different sensitivity of the tested strains and or method of extraction.
In this study A. nubica root bark extracts, phytochemical screening revealed the presence of terpenoids, phenols, tannins, flavonoids, and cardiac glycosides, which agrees with work done on A. nubica leaves with antimicrobial activities against both S. aureus and C. albicans.
Furthermore A. nubica seeds oil was found to be active against S. aureus, Bacillus subtilis and E. coli, but failed to exhibit activity against C. albicans.
Phytochemicals derived from every part of a plant including roots, stem, leaves, Flowers, fruits, seeds, etc., could vary in percent occurrence of detected compounds in different plant parts tested extracts, when using various solvents and or extraction procedures.
However some of the identified compounds could be artifacts due to the processes used in the extraction and or phytochemical analysis, furthermore, contaminants present in traces amounts in the organic solvents used in complex series of steps from plant part collection and storage, chemical treatments, chromatographic operations and instrumental analysis, any one of these steps provides the possibility for undesired artifacts, for example the possible occurrence of phthalates contaminants derived from utilization of plastic containers for solvents.
Sporadic studies have been reported on A. nubica especially root bark extracts one of which was reported on the antifungal activity against M. mycetomatis.
This study has determined that A. nubica Root bark crude ethanol 70% extracts and aqueous methanol fraction possess dose dependent antibacterial activity against S. aureus (ATCC 25923) at concentrations (75,150,300 and 600 mg/ml).
This study has determined that A. nubica Root bark crude ethanol 70% extract possess antifungal activity at concentrations 300 mg/ ml-600 mg/ml, against C. albicans clinical isolates.
GC/MS analysis confirmed the presence of the secondary metabolites that are potentially contributing to the observed biological activities in the A. nubica Root bark understudy are terpenes, tannins, flavonoids and saponins.
The results of this study provide a basis for further investigations of the A. nubica root bark extracts to serve as a source of novel antimicrobial agents.
• Further study of the antimicrobial activity of different parts of A. nubica (leaves, steam bark, flower) is recommended.
• Also, it is recommended to perform structure elucidation and mechanism of action analysis for the m ajor constituents A. nubica root bark extracts.
• Further studies on other potential pharmacological pro perties are recommended.
I am grateful to my supervisors Professor Elhadi M. Mohamed Ahmed, Associate Professor Abdelgadir Alamin Abdelgadir department of pharmacognosy for their continuous guidance advice effort and invertible suggestion throughout the research which allowed me to undertake this work.
My utmost gratitude to collogues Abubaker, Elhaj, Isra. Reem, Rowa, Saria, Wehad and Najat without their continuous support this study would not have been possible. I would also like to thank members of pharmacognosy laboratory Ameena, Nazek and Amna for helping to carry out my research.
I would also like to thank my friend Ibrahim Kamal for his help throughout this study.
Lastly, I would like to express my sincere appreciation to my parents especially my mother for encouraging and supporting me throughout the study.
I would like to declare and accept all responsibilities door this dissertation which represents my own work and efforts. The work was done in the laboratory of pharmacognosy and pharmaceutical microbiology, faculty of pharmacy, university of Gezira, Wad Medani city, Capital of Gezira, Sudan. Submitted to university of Gezira for partial fulfillments of the requirements for the award of the degree of master of pharmacy in pharmacognosy, department of pharmacognosy, faculty of pharmacy.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Journal of Pharmacognosy & Natural Products received 606 citations as per Google Scholar report