Research Article - (2020) Volume 3, Issue 2
Received: 15-Jun-2020
Published:
29-Jun-2020
, DOI: 10.37421/jcnn.2020.3.109
Citation: Nagy A, Mekhail, Ali R Rezai, Pragya B Gupta and W Porter McRoberts, et al. "Safety and Efficacy of Novel Epidural Clonidine Micropellets for Lumbosacral Radiculopathy". J Clin Neurol Neurosurg 11 (2020): 109. doi: 10.37421/jcnn.2020.3.109
Copyright: © 2020 Mekhail NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Chronic back and leg pain is a leading cause of disability and results in a significant health care expenditure. Options such as physical therapy, corticosteroids Epidural Injections (ESI) or spine surgery have limited long term benefit and may cause significant morbidity. Clonidine has anti-neuropathic pain and anti-inflammatory properties. A novel, slow-release biodegradable polymer (Poly (D, L-lactide) clonidine micropellets deliver sustained, high clonidine levels and may provide prolonged pain relief with minimal systemic side effects. Methods: A pilot multicenter investigation of the safety, pharmacokinetics (PK) and effectiveness of clonidine micropellets in patients with chronic lumbosacral radiculopathy. Three sequential cohorts of 18 subjects each received a single epidural injection of clonidine 0.325 mg, 0.975 mg or 1.95 mg (1, 3 or 6 micropellets, respectively) at the interlaminar epidural level that corresponds to the painful dermatomes. Clonidine PK was evaluated at multiple time points for 84 days. Paired t-tests were performed to examine changes from baseline within each cohort, and analysis of covariance evaluated differences between dose levels. NRS and RMS-Q disability scores were recorded at baseline and for one year follow up. Results: Peak plasma clonidine was observed 24h after injection and the mean elimination half-life ranged from 13 to 20 days. Statistically significant reduction in leg and back pain from baseline at all-time points up to 1 year was observed, in all cohorts as well as significant improvement in the RMS-Q at all timepoints. Adverse events and rescue medications were also recorded. Conclusion: Clonidine Micropellets would safely and slowly deliver clonidine over a prolonged period. Clonidine’s anti-neuropathic and anti-inflammatory effects could treat the radicular back and leg pain.
Pharmacokinetics (PK) • Clonidine micropellets • Epidural • Roland Morris Disability Scale (RMS-Q) • Low Back Pain (LBP) • Lower Extremity Pain (LEP) • Numeric Rating Scale (NRS)
Low Back Pain (LBP) with radicular Lower extremity pain (LEP) is relatively common with an estimated lifetime incidence ranging from 13%-40% [1]. Radicular pain is characterized by intense radiating leg pain that may be accompanied by sensory or motor disturbances or deep tendon reflex changes [1,2]. While many cases of acute sciatica resolve spontaneously, 30%-55% of patients have symptoms that persist and or recur beyond 1 year [3,4]. We reported a 47% rate of recurrence of LBP within 6 months to 2 years after the acute episode. The total cost of care for chronic or recurrent LBP or LEP was estimated at $100 billion in 2006, with two-thirds of this amount attributed to lost wages and decreased productivity [5].
Recent randomized trials failed to show any significant long-term improvement with anticonvulsant medications such as pregabalin with or without analgesic combinations [6-8]. ESI is the most common interventional pain management procedure performed to manage back and or leg pain, despite debatable evidence supporting its effectiveness for chronic LBP or LEP and despite clear increased risks and excessive cost associated with their repeated use. Such risks include; worsening of osteoporosis and diabetes, weight gain, peripheral edema, and other endocrine dysfunction [9,10]. Safe treatment interventions with lasting effects are needed to prevent acute radicular pain from becoming chronic and to manage the treatment- resistant chronic pain that contributes to high morbidity, healthcare and socioeconomic burden.
Clonidine is an alpha-2 adrenergic receptor agonist with both analgesic and anti-inflammatory properties [11-15]. Clonidine was approved in the US for hypertension in 197415 and is used for a variety of disorders, including in combination with opiates for the treatment of severe cancer pain that is not adequately relieved by opioid analgesics alone.
Several studies report reductions of post-operative pain following peri-operative administration of clonidine [16-19]. One study showed epidural administration of clonidine with local anesthetic produced comparable short-term pain relief, to epidural steroids with the same local anesthetic without serious adverse events or any signs of hypotension [20]. Findings supported by a trial comparing analgesic effects of steroids alone and in combination with low doses of clonidine (0.5 mcg/kg-1 mcg/kg) in lumbosacral radiculopathy [21].
A novel, slow-release formulation of clonidine, a “micropellet” consists of clonidine in a biodegradable polymer (Poly D, L-lactide) that is injected epidurally near the affected nerve roots. The micropellet has the potential to allow targeted delivery of high local concentrations for extended periods of time, thereby prolonging the clinical effects and reducing inflammation while reducing the risk of systemic side effects. The property was demonstrated by Beall and colleagues in a non-clinical model where they demonstrated sustained local nerve tissue levels for 12 weeks post-injection, despite absent systemic levels [22].
The primary objective of this study was to investigate the safety and Pharmacokinetics (PK) of epidural administration of the novel clonidine micropellets, in escalating sequential doses to subjects with chronic lumbosacral radiculopathy. A secondary objective was to assess the effect of the clonidine micropellets on leg and back pain and disability as measured by the Numeric Rating Scale (NRS) and Roland Morris Disability Scale Questionnaire (RMS-Q), respectively.
Study design and subjects
This open label, sequential, dose-escalation study was registered at ClinicalTrials.gov (NCT01917825) in July, 2013 and approved by the institutional review boards at 6 participating sites (7/2/2013). Enrollment period was from September 2013 until August 2015. The study was conducted in compliance with the Declaration of Helsinki principles for human medical research, and the principles of good clinical practice and data management [23]. Written informed consent was obtained from all study participants. Patients eligible for enrollment were ≥ 18 years with lumbosacral radiculopathy for a minimum of 6 weeks with: 1) unilateral pain radiating toward the buttock and extending down to the leg and/or feet; 2) disc protrusion, nonsequestered extrusion, or free fragment from L1 to S1 confirmed by magnetic resonance imaging and confirmed the clinical signs and symptoms of radiculopathy and (MRI); 3) mean NRS score ≥ 5 out of 10 at screening.
Subjects must have failed conservative therapy such as bed rest, physical therapy, medications, TENS, or manipulation for ≥ 6 weeks at time of screening. This duration was chosen in an attempt to capture patients with pain that did not resolve following a sufficient duration of time and who were considered high risk of progressing to chronic pain. This is a population that would typically be considered eligible for ESI.
Subjects were excluded for: 1) previous lumbar surgery, including vertebroplasty and kyphoplasty; 2) ESI or nerve block within 6 weeks; 3) current pain episode lasting ≥ 6 months; 4) more than 1 ESI within 6 months; 5) disc protrusion findings at more than one level on MRI; 6) symptomatic central stenosis; and 7) other pain generators greater than the radicular pain.
Study procedure
Subjects underwent a single interlaminar injection into the paramedian epidural space at the spinal level that corresponded to the painful dermatome. 2 ml of 1% Lidocaine solution was used to anesthetize the skin and the subcutaneous needle track. Clonidine Micropellets were injected from a pre-filled cartridge via a 3.5 inch, 18-gauge Tuohy needle under fluoroscopic guidance with contrast injection to confirm proper epidural needle placement before micropellets injection. Three sequential cohorts of 18 subjects each received a single injection of clonidine 0.325 mg (1 micropellet), 0.975 mg (3 micropellets), or 1.95 mg (6 micropellets).
Cohorts were enrolled sequentially to allow for Safety data to Day 42 to be reviewed for each cohort by an independent safety board before proceeding to the next cohort. Protocol-defined rescue pain medications were classified into 3 tiers and their use was recorded at baseline and after study treatment. Tier 1 rescue medications were acetaminophen, Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), or tramadol, tier 2 were hydrocodone or oxycodone, and tier 3 was immediate-release morphine sulfate.
Outcome measures
Safety was assessed by monitoring Adverse Events (AEs) for one year following the injection, with severity and relationship to the Clonidine Micropellets specified by investigators. Medical Dictionary for Drug Regulatory Affairs (MedDRA) version 16.0 was used to code the AEs. Additional safety evaluations included vital signs, orthostatic blood pressure and heart rate changes, physical examination, complete blood count, blood chemistry, and urinalysis.
The PK profile of clonidine was evaluated by plasma sampling preinjection and at 0.5, 1, 2, 4, 24 hours, and 7, 14, 21, 28, 35, 42, and 84 days post-injection.
Efficacy was assessed using the 11-point (0-10) NRS for pain [24]. Leg and back NRS scores were recorded twice daily (morning and afternoon) on paper diaries at least 5 days/week for 1 week during screening and for 12 weeks after the injection, and then weekly from Day 84. Physical functioning was assessed by the RMS-Q once during screening and weekly following treatment [25].
Statistical analysis
The sample size for each cohort was selected to facilitate collection of sufficient PK and safety data and obtain preliminary information on changes in ratings of pain intensity.
Safety was assessed by summarizing the incidence, relatedness, severity, and type of AEs as well as treatment-emergent changes in other safety assessments. All safety and tolerability analyses were descriptive and based on the Safety population. AEs were listed for all subjects and tabulated for each cohort.
The PK analysis calculated, summarized, and displayed Cmax, Cmin, Tmax, AUC0-t, and AUC0-∞ for the PK population. The activity analysis was based on the ITT population and examined changes from baseline in leg and back NRS scores within and between the 3 cohorts. Changes in physical functioning from baseline were assessed within and between cohorts based on RMS-Q.
Descriptive statistics were calculated for all variables, including frequency and percent responses for categorical variables and mean, median, and Standard Deviation (SD) for continuous variables. Paired t-tests were performed to examine changes from baseline within each cohort and analysis of covariance (with baseline values as a covariate) evaluated differences in response to treatment between the dose levels.
The Safety population included all subjects who received treatment, the PK population included all treated subjects who met all inclusion and no exclusion criteria, and the Intent-To-Treat (ITT) population consisted of subjects who received study treatment and provided ≥ 1 NRS or RMS-Q assessment. In this study, all populations were the same.
A total of 175 subjects consented for screening. 54 met the enrollment criteria and were enrolled into 3 sequential dosing cohorts and all 54 completed the study to Day 84 with 47 completing the study to day 364 (Figure 1). Seven subjects discontinued early, including 6 lost to follow-up and 1 due to an AE.
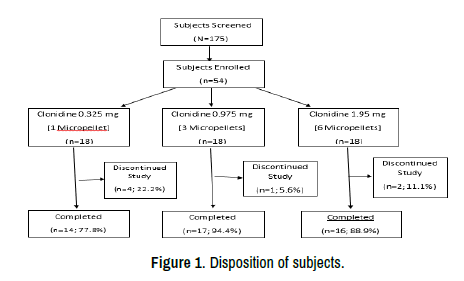
Figure 1. Disposition of subjects.
Overall, the cohorts were similar with respect to baseline demographic and clinical characteristics (Table 1). The majority of subjects were males in the 1- and 6-micropellet cohorts at 72.2% and 66.7%, respectively, compared with 66.7% female in the 3-pellet group. The mean age was 48.2 years (SD 12.1) and was similar across the cohorts. Almost all subjects were white at (98.1% overall). A higher percentage of subjects in the 3-pellets cohort were obese compared with the 1- and 6-pellet cohorts. More than half of the subjects reported taking NSAIDs (70.4%) or analgesics (57.4%) at baseline, with no notable differences in baseline medication use across cohorts.
| Cohort N (%) | 1 micropellet (n=18) |
3 micropellet (n=18) |
6 micropellet (n=18) |
Total (N=54) |
|---|---|---|---|---|
| Clonidine (mg) | 0.325 | 0.975 | 1.95 | N/A |
| Female/Male | 5/13 (27.8/72.2) | 12/6 (66.7/33.3) | 6/12 (33.3/66.7) | 23/31 (42.6/57.4) |
| Age-mean ± SD | 50.9 ± 12.2 | 47.4 ± 9 | 46.2 ± 14.5 | 48.2 ± 12.1 |
| White race | 18 (100.0) | 18 (100.0) | 17 (94.4) | 53 (98.1) |
| Ethnicity, n (%) Hispanic Y/N |
1/17 (5.6/94.4) | 0/18 (0/100) | 1/17 (5.6/94.4) | 2/52 (3.7/96.3) |
| BMI-mean ± SD | 28.6 ± 2.9 | 30.2 ± 6.1 | 27.8 ± 5.5 | 28.9 ± 5.1 |
| BMI<18.5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BMI 18.5-24.9 | 2 (11.1) | 4 (22.2) | 5 (27.8) | 11 (20.4) |
| BMI 25.0-29.9 | 11 (61.1) | 5 (27.8) | 7 (38.9) | 23 (42.6) |
| BMI≥30 | 5 (27.8) | 9 (50.0) | 6 (33.3) | 20 (37.0) |
| NSAIDS/an | 12 (66.7) | 12 (66.7) | 14 (77.8) | 38 (70.4) |
| Analgesics | 11 (61.1) | 10 (55.6) | 10 (55.6) | 31 (57.4) |
| RAS-acting agents | 3 (16.7) | 3 (16.7) | 2 (11.1) | 8 (14.8) |
| Muscle relaxants | 2 (11.1) | 2 (11.1) | 4 (22.2) | 8 (14.8) |
| Acid-related disorders agents | 3 (16.7) | 1 (5.6) | 3 (16.7) | 7 (13.0) |
| Lipid-modifying agents | 3 (16.7) | 1 (5.6) | 3 (16.7) | 7 (13.0) |
| Vitamins | 5 (27.8) | 1 (5.6) | 1 (5.6) | 7 (13.0) |
| Tier 1 | 17 (94.4) | 13 (72.2) | 14 (77.8) | 44 (81.5) |
| Tier 2 | 3 (16.7) | 3 (16.7) | 3 (16.7) | 9 (16.7) |
BMI: Basal Metabolic Index. Tier 1 medications included acetaminophen, nonsteroidal antiinflammatory drugs (NSAIDS), or tramadol. RAS: Renin-Angiotensin system Tier 2 medications included hydrocodone or oxycodone. BMI: Body M
At baseline, tier 1 rescue medications were used by 81.5% of subjects while less than 20% reported use of tier 2 rescue medications. The most frequently reported post-treatment medications unrelated to the injection procedure were analgesics (79.6%), NSAIDs (64.8%), psycholeptics (51.9%), systemic corticosteroids (42.6%), systemic antibacterials (35.2%), and muscle relaxants (31.5%).
Safety
At least 1 AE was reported for 85.2% of subjects (Table 2), with 99% considered unlikely or not related to treatment. Most AEs were mild (66.7%) or moderate (23.2%) in intensity. The most frequently reported AEs were newly emerging or worsening back pain (35.2%), pain in extremity (31.5%), and headache (24.1%) and all were more common in the 1-pellet cohort. Details of all AEs are summarized in Table 3. Nasopharyngitis, muscle spasms, and procedural pain were reported for >10.0% of subjects overall with no apparent relationship to dose.
| Cohort N (%) | 1 micropellet (n=18) |
3 micropellet (n=18) |
6 micropellet (n=18) |
Total (N=54) |
|---|---|---|---|---|
| Clonidine (mg) | 0.325 | 0.975 | 1.95 | N/A |
| By day 29 | 13 (72.2) | 12 (66.7) | 9 (50.0) | 34 (63.0) |
| Days: 30-85 | 11 (61.1) | 7 (38.9) | 4 (22.2) | 22 (40.7) |
| Days: 86-183 | 9 (50.0) | 4 (22.2) | 6 (33.3) | 19 (35.2) |
| Days: 184-385 | 8 (44.4) | 6 (33.3) | 8 (44.4) | 22 (40.7) |
| Mild AE | 5 (27.8) | 9 (50.0) | 7 (38.9) | 21 (38.9) |
| Moderate AE | 8 (44.4) | 2 (11.1) | 2 (11.1) | 12 (22.2) |
| Severe AE | 2 (11.1) | 5 (27.8) | 6 (33.3) | 13 (24.1) |
| Not related AE | 12 (66.7) | 9 (50.0) | 10 (55.6) | 31 (57.4) |
| Unlikely related AE | 2 (11.1) | 6 (33.3) | 5 (27.8) | 13 (24.1) |
| Possibly/probably/ definitely related AE |
1 (5.6) | 1 (5.6) | 0 (0.0) | 2 (3.7) |
| SAEs | 2 (11.1) | 2 (11.1) | 2 (11.1) | 6 (11.1) |
| Discontinued due to AE | 1 (5.6) | 0 (0.0) | 0 (0.0) | 1 (1.9) |
| Dead due to AE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cohort N (%) | 1 micropellet (n=18) |
3 micropellet (n=18) |
6 micropellet (n=18) |
Total (N=54) |
|---|---|---|---|---|
| Clonidine (mg) | 0.325 | 0.975 | 1.95 | N/A |
| Back pain | 11 (61.1) | 5 (27.8) | 3 (16.7) | 19 (35.2) |
| Pain in extremity | 9 (50.0) | 3 (16.7) | 5 (27.8) | 17 (31.5) |
| Headache | 8 (44.4) | 4 (22.2) | 1 (5.6) | 13 (24.1) |
| Nasopharyngitis | 13 (24.1) | 3 (16.7) | 4 (22.2) | 8 (14.8) |
| Muscle spasms | 1 (5.6) | 4 (22.2) | 2 (11.1) | 7 (13.0) |
| Procedural pain | 3 (16.7) | 2 (11.1) | 2 (11.1) | 7 (13.0) |
| Arthralgia | 1 (5.6) | 2 (11.1) | 1 (5.6) | 4 (7.4) |
| Fall | 2 (11.1) | 0 (0.0) | 2 (11.1) | 4 (7.4) |
| Hypertension | 0 (0.0) | 1 (5.6) | 3 (16.7) | 4 (7.4) |
| Nausea | 0 (0.0) | 2 (11.1) | 2 (11.1) | 4 (7.4) |
| Constipation | 1 (5.6) | 2 (11.1) | 0 (0.0) | 3 (5.6) |
| Influenza | 0 (0.0) | 1 (5.6) | 2 (11.1) | 3 (5.6) |
| Insomnia | 3 (16.7) | 0 (0.0) | 0 (0.0) | 3 (5.6) |
| Musculoskeletal pain | 0 (0.0) | 0 (0.0) | 3 (16.7) | 3 (5.6) |
| Sinusitis | 1 (5.6) | 2 (11.1) | 0 (0.0) | 3 (5.6) |
No deaths occurred during the study and 6 Serious Adverse Events (SAEs) were reported in 6 subjects (11.1%), 2 in each cohort and all considered unrelated to treatment. In the 1-micropellet group, 1 subject was diagnosed with osteoarthritis (Day 78) that required hip replacement surgery and another subject was diagnosed with L4-L5 instability (Day 282), which required spine surgery. One subject in the 3-micropellet group developed an incarcerated umbilical hernia that required repair (Day 207) and another was diagnosed with L4-L5 disc herniation (Day 105) requiring spine surgery. The 2 SAEs in the 6-micropellet group were breast cancer (Day 358) and a fall (Day 291) requiring orthopedic surgery.
Regarding AEs of special interest, procedural dizziness (during blood draw) was reported by 1 subject in the 3-micropellet group on Day 22 and considered unrelated to treatment. Two mild events of dizziness were reported with 1 event in the 6-micropellet group on Day 3 considered unlikely related and one in the 1-micropellet group on Day 7 considered unrelated. A single event of orthostatic hypotension occurred in the 1-micropellet group on Day 36 and considered unlikely related. One mild event of transient (non-orthostatic) hypotension occurred in the 3-micropellet group on Day 6 and was also considered unrelated. There were no infections considered related to the procedure or the Clonidine Micropellets.
Pharmacokinetics
The peak plasma clonidine concentration was observed 24 hours following the injection in each cohort, with a Cmax of 59.8 pg/mL in the 1-pellet group, 136.6 pg/mL in the 3-pellet group, and 250.7 in the 6-pellet group. The mean elimination half-life ranged from 13 to 20 days.
Change from baseline in the numeric rating scale of radicular leg and back pain
Although the protocol called for a minimum of 6-week symptom duration at baseline, the overall average symptom duration of enrolled subjects was 20.4 weeks. NRS scores measured in the morning and evening demonstrated a similar pattern of pain reduction, with the evening and morning scores presented in (Figure 2a and 2b).
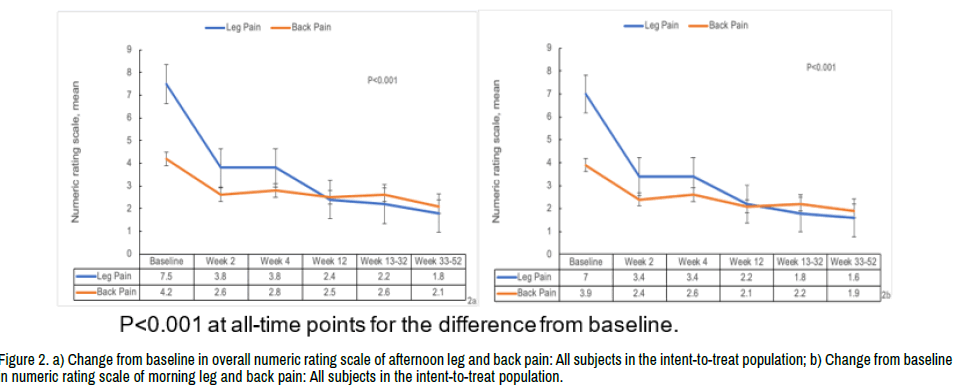
Figure 2. a) Change from baseline in overall numeric rating scale of afternoon leg and back pain: All subjects in the intent-to-treat population; b) Change from baseline in numeric rating scale of morning leg and back pain: All subjects in the intent-to-treat population.
The overall mean baseline evening leg pain score was 7.49 (SD 1.22; Figure 2a), which was similar between the 3 dose groups at 7.43 (SD 0.92), 7.6 (SD 1.35), and 7.39 (SD 1.38) in the 1-, 3-, and 6-micropellet cohorts, respectively. There was a statistically significant reduction in the back pain from baseline evident within 1 day of injection and maintained at all postbaseline assessment time points in each of the 3 cohorts and similar reductions in pain reported within each dose group. Scores in each group decreased significantly (49% overall) during the first 2 weeks following the micropellets injection (p<0.001) with a slower, but consistent, overall decrease in pain until the end of the study (Figure 2a and 2b). Similar reductions in pain were reported for all 3 dose groups.
The mean baseline afternoon back pain rating was 4.17 (SD 2.73) for the 44 subjects with baseline leg pain and scores were similar between the 3 dose groups. There was a statistically significant reduction in back pain from baseline to all post-baseline assessment time points in each of the 3 cohorts and similar reductions in pain reported within each dose group (Figure 2).
Roland morris disability scale
Baseline scores for the RMS-Q were comparable across the 3 dose groups with 14.6 (SD 4.84) in the 1-micropellet group, 15.3 (SD 4.21) for the 3-micropellet group, and 14.2 (SD 4.80) in the 6-micropellet group. The overall mean score decreased from a baseline of 14.7 (SD 4.56) to 8.7 (SD 6.23) on Day 8 with further declines to 6.4 (SD 7.06) on Day 43, and 4.2 (SD 6.91) at end of study on Day 364 (Figure 3). All cohorts performed similarly and the changes were statistically significant overall and within each dose group at all timepoints.
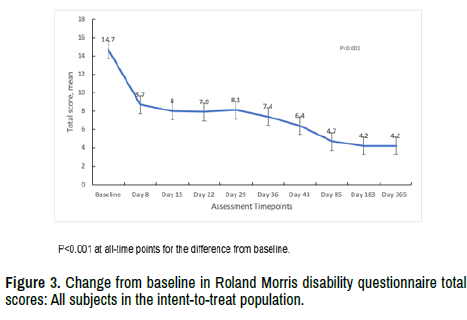
Figure 3. Change from baseline in Roland Morris disability questionnaire total scores: All subjects in the intent-to-treat population.
Rescue medications and nonpharmaceutical therapies following clonidine micropellets injections
Nine subjects (50%) in the 1-pellet cohort were prescribed rescue medications compared with 6 (33.3%) in the 3-pellet group and 7 (38.9%) in the 6-pellet group. The most frequently used rescue medications were analgesics (35.2%), NSAIDs (22.2%), and systemic corticosteroids (11.1%) with no notable differences across cohorts. Fifteen subjects (27.8%) received nonpharmaceutical treatment with no dose-related pattern.
Low back pain with or without radiculopathy is a leading cause of disability in the US25 and is associated with high healthcare resource utilization as well as high direct and indirect costs estimated to exceed $100 B per year in the US, over half of which can be attributed to lost productivity [5] Current guidelines recommend conservative therapies and noninvasive interventions, [9] although a substantial proportion of patients experience refractory or recurrent pain often requiring intervention.
This study establishes the safety of a novel, slow-release, targeted, epidurally delivered formulation of clonidine in a biodegradable polymer “micropellet”, in patients with sciatica and suggests that this formulation might provide an effective intervention for the relief of pain related to sciatica. There were no significant safety concerns related to the Clonidine Micropellets, including the absence of significant injection-site reactions, episodes of sedation or hypotension, or rebound hypertension. This favorable safety profile is consistent with the PK data that revealed peak systemic levels (250 pg/ml at the 6-pellet, 1.95 mg dose) that were substantially less than levels associated with centrally mediated hypotensive effect of clonidine (0.5 ng/ml-2.0 ng/ml) [15] We also observed a slow and gradual decline in clonidine levels over approximately 35 days at the highest dose, which mitigates against rebound effects. Notably, there were immediate (within 1 day), clinically significant improvements in patient-reported levels of leg and back pain as well as disability improvements, with effects maintained for 12 months.
Our study demonstrated onset of pain relief within 1 day of injection of clonidine micropellets in a population with baseline symptom duration >20 weeks, with pain continuing to improve over approximately 4-6 weeks (49% reduction at 4 weeks) and results sustained to 1 year (76% reduction). The slow-release micropellet formulation allowed clonidine to be detected in the systemic circulation within 1 day and remain detectable for up to 35 days, which is consistent with the rapid onset and gradual increase in analgesic effect seen over the first 4-6 weeks likely due to immediate analgesic effect followed by longer term reduction of inflammation, respectively. There was a high percentage of responders, with 80% experiencing at least a 30% reduction in pain at 12 weeks.
The majority of patients with acute sciatica will recover within a few weeks. The population studied in this trial was selected because their symptoms lasted beyond the acute phase, going into the sub-acute and chronic stage [9]. These subjects were likely experiencing neuropathic/inflammatory pain resulting from upregulation of production of inflammatory mediators irritating the nerve roots and the dorsal root ganglia. Results of this trial support the hypothesis that clonidine’s anti-inflammatory/anti-neuropathic effects may reverse the neuropathic inflammation at the DRG and nerve roots, resulting in sustained pain relief. The favorable effects seen with the micropellet formulation warrant further randomized controlled studies.
Notably, each of the 3 doses demonstrated activity on reducing sciatica pain which may indicate that each dose delivered local clonidine levels that exceeded the concentrations required for efficacy. It is possible that evaluation of a lower dose might have revealed a dose-response relationship and it is notable that the 0.325 mg dose resulted in a higher reported incidence of adverse events of back and extremity pain, which could be an indication of lower efficacy at this dose level.
Limitations of this study include the open-label design and lack of a control group, which limits our ability to confirm the treatment effect. We believe the observed time of onset, magnitude of impact and duration of action support the conclusion that these effects can be attributed to the pharmacologic action of clonidine. Interpretation of these findings is also limited by the small sample size, which was not intended to support a full assessment of efficacy. An additional limitation was the allowance of liberal use of post-treatment rescue medications which introduces the risk of bias due to inconsistencies in thresholds for use and reporting across sites and investigators.
An ongoing prospective, multicenter, phase 3, sham-controlled, randomized, double-blind trial studying the 0.975 mg (3 micropellet) dose in patients with lumbosacral radiculopathy (RePRIEVE-CM), will assess improvement in pain as well as several measures of function and quality of life. The study will assess the primary endpoint of improvement in pain intensity at 30 days and subjects can choose to enroll in a long-term follow-up to assess safety and maintenance of pain relief at 1 year.
• Nagy A. Mekhail: Contributed to the design of the study, analysis and interpretation of data and writing of the final manuscript
• Ali R. Rezai: Contributed to the analysis and interpretation of data and writing of the final manuscript
• Pragya B. Gupta: Contributed to the design and execution of the study and to the writing of the final manuscript
• W Porter McRoberts: Contributed to the design and execution of the study, analysis and interpretation of data and writing of the final manuscript
• Gregory J. Fiore: Contributed to the analysis and interpretation of data and writing of the final manuscript
• Bryan A. Jones: Contributed to the analysis and interpretation of data and writing of the final manuscript
• Lou-Anne G. Acevedo-Moreno: Contributed to the writing of the final manuscript
• Chris J. Gilligan: Contributed to the analysis and interpretation of data and writing of the final manuscript
• Ramsin M. Benyamin: Contributed to the design and execution of the study, analysis and interpretation of data and writing of the final manuscript
Phase II trial was funded by Medtronic Inc. and registered at ClinicalTrials. gov (NCT01917825) in July, 2013.
Carole Alison Chrvala, PhD, Health Matters, Inc., is acknowledged for her assistance with some aspects of the manuscript preparation.
Journal of Clinical Neurology and Neurosurgery received 2 citations as per Google Scholar report