Research Article - (2022) Volume 8, Issue 5
Received: 23-May-2022, Manuscript No. jpnp-22-64673;
Editor assigned: 25-May-2022, Pre QC No. P-64673;
Reviewed: 10-Jun-2022, QC No. Q-64673;
Revised: 15-Jun-2022, Manuscript No. R-64673;
Published:
22-Jun-2022
, DOI: 10.37421/2472-0992.22. 8.185
Citation: Aljaziri, Maryam A., Dina Hajjar, Abdul-Hamid Emwas
and Arwa A. Makki et al. “Phytochemical Influence of Salvia Officinalis L. from
Saudi Arabia and Palestine on Oxidation and HIV Viral Load” J Pharmacogn Nat
Prod 8 (2022): 185.
Copyright: © 2022 Aljaziri MA, et al. This is an open-access article distributed
under the terms of the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.
Competing interests: The authors have declared that no competing interests exist.
Sources of funding : None
Background: This study sought to identify and characterize the diverse flavonoids and phytochemical compounds in Saudi and Palestinian Salvia officinalis (sage) leaf extracts, and to evaluate their ability to inhibit protease enzymes, and thus their potential for use in antiretroviral drug discovery and development. We also explored sage’s ability to scavenge radicals; antioxidants derived from Lamiaceae plants play an important role in natural medicine.
Methods: The crude extract of methanol and dichloromethane solvents from Saudi and Palestinian Salvia officinalis leaves (sage) were in vitro screened for inhibitory activity against HIV-1 protease. We examined antioxidant capacity against the synthetic radicals of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), identified the chemical constituents of the extracts by gas chromatography–mass spectrometry, ultrahigh- pressure liquid chromatography and nuclear magnetic resonance, and determined the diversity of flavonoids by liquid chromatography.
Results: Regarding the antiviral results, both Saudi and Palestinian sage leaf extracts significantly inhibited HIV-1 PR activity when compared to the vehicle control which was approximately (25.1 ± 10.6) μg/mL. The investigation of antioxidant capability revealed significant scavenging of synthetic ABTS radicals by Palestinian Salvia officinalis (sage) leaf extracts compared to the Saudi sage leaf extracts. Chemical analyses revealed a wide variety of secondary and primary compounds and flavonoids which were compared.
Conclusion: In this study we have investigated and revealed a variety of biological compounds that contribute to the inhibition of oxidative stress and protease enzyme. The results provide promising and beneficial data for the discovery and development of novel drugs against oxidation and HIV/AIDS.
Salvia officinalis • Palestinian sage leaves • Antioxidants • Antivirals• HIV/AIDS • Methanol/dichloromethane extract • Flavonoids • Protease
Methanol/Dcm Extract: Methanol/Dichloromethane Extract; ART: Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; MeOH: Methanol; NMR: Nuclear Magnetic Resonance; CDCl3: Chloroform; GCMS: Gas Chromatography–Mass Spectrometry; UHPLC: Ultra- High-Pressure Liquid Chromatography; ESI: Electrospray Ionization; ABTS•+: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); RS: Radical Scavenging; DMSO: Dimethyl Sulfoxide; PR: Protease; RFU: Relative Fluorescence Unit.
Salvia officinalis L. (commonly referred to as sage) is a member of the Lamiaceae family, belonging to the Nepetoideae subfamily, Mentheae tribe and Salvia genus. S. officinalis L. is commonly found in south-east Asia, in Europe (primarily around the Mediterranean) and in Central and South America [1].
The Kingdom of Saudi Arabia (KSA) is home to a wide variety of plants and is among the countries that cultivate sage. However, extant data regarding sage is from plants of Non-Saudi origin [2]. Multiple published studies have indicated the potential benefits of sage for public health; this is due to its biologically effective components [3], which include antioxidants. Half of these compounds are phenols or flavonoids, which play an important role in the prevention of disease [4].
Notably, half of sage’s phenolic compounds are flavonoids that present as aglycone, glycosides and methylated derivatives [5]. Flavonoids are structures of 15 carbon units C6-C3-C6 attached to two benzene rings (A and B), connected by a three-carbon pyran ring (C). The site of the catechol B-ring on the pyran C-ring, and the number and position of hydroxyl groups on the catechol group of the B-ring, affect the flavonoids’ antioxidant capability [6]; the functional hydroxyl group in flavonoids acts to inhibit oxidative stress by suppressing free radicals and chelating metal ions [7]. Sage leaves are a source of renewable biomass that could helpfully be converted into value‑added chemicals for medicine. Currently, herbal products with antiviral properties are of interest to many scientists, due to the modern capacity for viral and other microorganisms to resist drug treatments [8], and because the limitations of Antiretroviral (ART) treatments for human immunodeficiency virus (HIV) drive a continuous search for more efficient anti‐ HIV drugs. Medical communications indicate that some medicinal plants have significantly high antiviral potential; herbal medicines have been found very efficacious for inhibiting retroviruses and may have a place in the treatment of HIV/AIDS [9]. Thus, the present study seeks to reveal the flavonoid content of methanolic dichloromethane (DCM) extractions from sage plants grown in Tabuk of KSA and Bani Naim of Palestine, with a survey of the entire chemical profile, studying the chemical composition and evaluating the bioactive effects and activity of both plants on oxidative stress and human immunodeficiency virus (HIV).
Plant identification and extraction
Dried Salvia officinalis L. of Saudi origin were purchased from a local market in Jeddah, KSA (Source origin: Tabuk), and dried Palestinian Salvia officinalis L. were obtained from a plantation in Palestine (Source origin: Bani Naim). These were each ground into a fine homogeneous powder using a mortar, pestle, and grinder. Thereafter, they were extracted in two phases; first by using dichloromethane: methanol (1:1) by maceration in 1.5 ml, overnight at room temperature (12 hours), and then by vortex and centrifuge at 14680 rpm (full speed) for 2 min. Next, the extracts were transferred to a fresh 2 ml Eppendorf tube, and 0.4 ml of methanol was added to the remaining marc and vortexed to ensure a complete mixture, then extracted for 30 min at room temperature, then centrifuged at 14680 rpm (full speed) for 2 min. Thereafter, the extract was transferred to the DCM/MeOH phase in the manner described in reference [10], and stored at -20°C until the experiment began.
NMR analysis
Nuclear magnetic resonance (NMR) spectroscopy is a versatile analytical tool that has long been used for the identification and quantification of molecules [11-13]. Moreover, NMR can also be used for structural elucidation and to probe molecular dynamics. Besides its widespread application in the liquid phase, NMR spectroscopy can be also successfully used with solid states [14-16]. In the current study, both liquid and solid-state NMR (ssNMR) was used to investigate the chemical composition of the Saudi and Palestinian Salvia officinalis leaves.
One of the most important advantages of solid-state NMR is the fact that one can examine the chemical composition without any extraction; this may enhance the authenticity and integrity of studying the sample, in terms of its intrinsic chemical composition.
Solid-state – NMR: The 13°C solid-state NMR spectra were acquired using a Bruker Avance III 400 spectrometer equipped with a 4 mm double resonance Magic Angle Spinning (MAS) probe (Bruker BioSpin, Rheinstetten, Germany). To create comparable data, all processes were carried out using the same parameters and under the same conditions. Measurements were made with a 12 kHz to 14 kHz spinning rate using a cross-polarization experiment cp pulse program from the Bruker pulse library, with 3 ms contact time and a recycle delay time of 5 s. To achieve a high signal-to-noise ratio, the spectra were recorded by collecting at least 2 k scans. An adamantane standard sample was used as an external reference, where the chemical shifts signal was fixed at 37.7 ppm for 13°C spectra. Bruker Topspin 3.5pl7 software (Bruker BioSpin, Rheinstetten, Germany) was used for both data collection and spectral post-processing.
Solution - NMR 500 Hz: The dried extracts were dissolved in 600 μL of CDCl3 and then 550 μL were transferred to 5 mm NMR tubes. Dimensional NMR spectra were acquired using a Bruker Avance III 500 MHz NMR spectrometer equipped with a multinuclear Bruker CP Cryo-Probe (BrukerBioSpin, Rheinstetten, Germany). Operation was at 500 MHz using the following conditions: 128 scans with a recycle delay time of 5 s, using one pulse sequence through a standard (zg) program from the Bruker pulse library. Chemical shifts were corrected using the TMS signal at 0.0 pp, as an internal chemical shift reference.
Solution - NMR 800 Hz: NMR spectra were recorded through a Bruker 800 MHz Avance III NMR spectrometer, a Bruker 800 MHz SB liquid NMR instrument equipped with a 5 mm TCI cooling probe with a Z-axis gradient (Bruker BioSpin, Rheinstetten, Germany). Operation comprised 16 scans with 4 dummy scans and 2 seconds of recycling delay for 1H-proton detection. The dried extracts were dissolved in 600 μl of DMSO solvent, vigorously vortexed until the sample dissolved completely, and then transferred to 5 mm NMR tubes for analysis.
Gas Chromatography–Mass Spectrometry (GC–MS) analysis
The instrumentation used for GC–MS was an Agilent 7010B Triple Quadrupole GC/MS and flame ionization detector, coupled with a 7890B gas chromograph system (Agilent Technologies Inc., Santa Clara, CA, USA). The analysis was performed under the following conditions: DB-5 TR 5MS 30 m capillary column, the oven temperature was programmed from 70°C, carrier gas: helium, EI nominal mass instrument. Operation was in full scan mode: speed 2 scans per sec, 1000 resolving power, resolution 0.7 Da, mass range from 35–700. The Salvia officinalis derivatized extract injected was 1 μL.
UHPLC ID–X Orbitrap mass spectrometry analysis
Ultra-high performance liquid chromatography (UHPLC) analyses of Saudi and Palestinian Salvia officinalis leaves were performed using UHPLC, with an Orbitrap IQ-X mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Separations were carried out on Acquity CSH 100 × 2.1 mm, 1.7 μm express C18 column (Agilent Technologies Inc., Santa Clara, CA, USA). The mobile phase was composed of two solvents: 100% water + (0.1% formic acid) (A) and 100% acetonitrile + (0.1% formic acid) (B); injection volume was 5 μL, and the flow rate was set to 0.5 mL/min. Thermo Scientific’s XcaliburTM software (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to process and collect data. For the MS conditions, acquired under positive ionization mode (ESI+), the following parameters were applied: vaporized temperature 100ºC, voltage 3500 V, sheath gas 30, auxiliary gas 15, ion source fragmentation 35V, and capillary temperature 300ºC.
Flavonoid identification
The determination of flavonoids was performed with DCM/MeOH extracts through Orbitrap IQ-X mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) following the method previously described.
In vitro antioxidant bioassay
The ABTS·+ free radical scavenging assay was performed according to the description given in reference [17]. The ABTS·+ solution was prepared by reacting 5 mL of ABTS (7 mM) with 88 μL of potassium persulfate (140 mM) and incubated at room temperature in the dark for 12-16 h. It was then diluted with methanol until it reached a final absorbance of 0.7 at 734 nm; 10 μL were added to a 96-well microplate from different concentrations of extract (50, 100, 300, 500, 600) mg/mL and 190 μL of methanolic ABTS·+ solution. This was followed by incubation in dark conditions at room temperature for 5-10 min. The absorbance was read at 734 nm, ascorbic acid was selected as a positive control, and the results were reported as IC50 value, the radical scavenger percentage being calculated by the following formula:
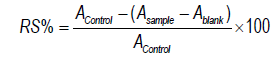
Where A sample, A blank, and A control were the absorbance of sample, blank, and control, respectively.
In vitro antiviral (HIV-1) bioassay
HIV-1 protease activity was calibrated according to the prescribed method for use with the SensoLyte® 520 HIV-1 protease fluorimetric assay kit (Cat No. AS-71147, AnaSpec, Fremont, CA, USA). A 40 μL sample of diluted HIV-1 PR (Cat# AS-72028-5) was reacted with 10 μL of DMSO solvent of test extracts samples at (100 and 500) mg/mL concentrations. The positive controls were 40 μl of diluted HIV-1 protease and 10 μL of 1x assay buffer, inhibitor controls were 40 μL diluted HIV-1 protease and 10 μL of pepstatin A, vehicle controls were 40 μL of diluted HIV-1 protease and 10 μL of DMSO, substrate controls were 10 μL substrate and 40 μL of 1X assay buffer in the 96-well microplates with flat-bottom, nonbinding surfaces (Cat No. CLS3600). These were vortexed gently and incubated in dark conditions for 15 min at room temperature, then 50 μL substrate of HIV-1 PR was added to the wells followed by further incubation for 60 min, after which 50 μL stop solution (Cat# AS71147) was added, in order to end the reaction. The fluorescence intensity of the HIV-1 protease activity was measured at an excitation (Ex) wavelength of 485 nm and emission (Em) wavelength of 528 nm per minute, using an end-point reading. The reading relative fluorescence unit was calculated by the following equation:

Where A = absorbance. A graph was generated by plotting inhibition percentage values against extract concentrations. The half-maximal inhibitory concentration (IC50) was calculated from the percentage value.
There is currently considerable interest in study of the structural and electronic properties of magnetic systems in both solution and in solid states [18]. Nuclear magnetic resonance (NMR) spectroscopy is a potent analytical procedure that has long been held indispensable for the illustration of structures in natural products. Despite the low sensitivity of NMR, it has the advantage in terms of observing the local magnetic fields around the atomic nuclei of the individual material and is quantitatively accurate. It also provides analysis of difficult-to-ionize compounds without the need for derivatization. In addition, NMR is fundamental for the location of structures in unknown compounds; for structural characterization of both sample extracts the 13C spectra on 400Hz were revealed as shown in in Figure 1. The chemical shifts are shown corresponding regions. Correlations were (δ 15- δ 55 ppm secondary alkyl (methylene), δ 65- δ 90 ppm alkynyl RC≡CR, δ 100- δ 150 ppm alkenyl R2C=CR2 and, δ 170 ppm for aromatic (phenyl ring C)) in Saudi and Palestinian Salvia officinalis (leaf) extracts. While proton spectra tend to be complicated in chemical shifts of smaller semblance than the carbon signals, the 1H proton spectrum was recorded at 500Hz; the signals at δ 0.9- δ 1.2 ppm for R-CH3 alkyl (methyl), δ1.5- δ2 ppm were assigned at alkyl (methine) R3C-H, δ 3.7 ppm typical for CH3CH2OH and aromatic signals between δ 7- δ 8 ppm in S and P Salvia officinalis L. extracts as illustrated in Figure 2. Further 1H-proton signals detected by NMR 800Hz were conformable in both extracts, as explained in Figure 3. The peaks between δ 0.9 ppm- δ 1.2 ppm for R-CH3 alkyl (methyl), signals between δ1.5- δ2 ppm for alkyl (methine) R3C-H, the signal at region δ 2.5 ppm for (CH3CH2)3N and signal at region δ 3.3 ppm for (CH3O)2CH, the results of the signals reported in NMR confirm the presence of several biologically active compounds. Thirty (30) are reported, of different phytochemical prospection, from primary metabolites using GCMS in Saudi Salvia officinalis L. derivatives. The GC-MS chromatogram of the 30 peaks of the compounds detected is shown in Figure 4 and Table 1, while thirty tree (33) peaks in Palestinian Salvia officinalis L. are reported, as shown in Figure 5 and Table 2. Thirteen (13) combined primary metabolites were detected in both polar (DCM/MeOH) extracts of Salvia officinalis L. including L-camphor, glycerol, α-terpineol, α-terpinyl acetate, caryophyllene, aromandendrene, humulene, caryophyllene oxide, L-arabitol, d-mannose, d-glucose, D-mannitol and myo-inositol. According to chromatogram, glycerol, D-(-)-fructose, d-mannose,and d-glucose were the most abundant compounds and indicated the highest peaks in Saudi Salvia officinalis L., while glycerol, α-terpineol, caryophyllene, d-glucose, dihydrocodeine and trimethylsilyl 7-isopropyl-1,1-dimethyl-5,6-bis((trimethylsilyl)oxy)1,2,3,4,4a,9,10,10aoctahydrophenanthrene- 4a-carboxylate were the most abundant compounds in Palestinian Salvia officinalis L. Similar compounds and results were found with hexane solvent from oil of Salvia guaranitica L. and supercritical (SCCO2) extracts of Salvia officinalis L. by JokiÄ? S, et al. [19].
In the positive ion mode mass spectrum obtained via UHPLC, 25 secondary metabolites were obtained from Saudi sage. The most abundant compound was desotamide E, followed by 2-[(3,3-dimethyl-2-oxobutyl) thio]- 6-oxo-4-pyridin-3-yl-1,6-dihydropyrimidine-5-carbonitrile as shown in Figure 6 and Table 3. Furthermore, 22 secondary metabolites were obtained from Palestinian sage where, among the derivatives, secondary metabolites piperalin and 1,4-androstadiene-3,17-dione were the most dominant phytochemical compounds, as shown in Figure 7 and Table 4. The natural compound desotamide was first discovered by Miao S, et al. [20], and shows antimicrobial activity against gram-positive organisms. A study in Taiwan Iâ?Chen L, et al. [21] reported the presence of a similar compound of 1,4-androstadiene-3,17-dione, which is a steroid hormone (4-androstene-3,17-dione), in ddH2O-prepared egg extracts under positive mode. Metabolites of 12 and 13 in Palestinian sage showed a prevalent fragment ion at m/z 315.0, referencing a relationship between them. To further investigate the phytochemical profile of both extracts, flavonoids were identified and characterized in methanol/DCM extracts. The Saudi sage showed more diversity in flavonoid content; 16 flavonoid
Figure 1. Stack plot of 13C NMR spectra of Saudi sage (in red) and Palestinian sage (in blue). The two spectra are similar, with slightly higher aliphatic carbon ratio in Palestinian sage.
| No. | Identification | Rt [min] |
|---|---|---|
| 1 | L-camphor | 13.06 |
| 2 | L-Proline, TMS | 13.37 |
| 3 | Borneol TMS | 14.53 |
| 4 | Glycerol 3TMS | 15.34 |
| 5 | Linolool oxide, TMS | 16.25 |
| 6 | (S)-(-)-α-Terpineol TMS | 16.89 |
| 7 | α-Terpinyl acetate | 17.73 |
| 8 | Caryophyllene | 19.46 |
| 9 | Aromandendrene | 19.89 |
| 10 | Humulene | 20.27 |
| 11 | meso-Erythritol, 4TMS | 20.43 |
| 12 | Caryophyllene oxide | 23.03 |
| 13 | L-(-)-Arabitol, 5TMS | 24.46 |
| 14 | ÃÂ? D-(-)-Fructofuranose, pentakis(trimethylsilyl) ether (isomer 1) | 26.00 |
| 15 | D-Psicose, pentakis(trimethylsilyl) ether, methyloxime (anti) | 26.88 |
| 16 | D-(-)-Fructose, pentakis(trimethylsilyl) ether, methyloxime (anti) | 27.16 |
| 17 | D-(-)-Fructose, pentakis(trimethylsilyl) ether, methyloxime (syn) | 27.34 |
| 18 | d-Mannose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- | 27.40 |
| 19 | d-Galactose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- | 27.49 |
| 20 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- | 27.59 |
| 21 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1E)- | 27.93 |
| 22 | D-Mannitol | 28.04 |
| 23 | α-D-(+)-Talopyranose, 5TMS | 29.04 |
| 24 | Myo-Inositol 6TMS | 30.73 |
| 25 | Farnesol, TMS | 32.07 |
| 26 | α-Linolenic acid | 33.25 |
| 27 | Kolavelool | 35.17 |
| 28 | Sucrose, 8TMS | 38.04 |
| 29 | Ursolic acid 2TMS | 48.96 |
| 30 | Ursolic acid 2TMS (isomer) | 49.59 |
compounds were reported, compared to 11 flavonoids in its Palestinian counterpart. These results are summarized in Tables 5 and 6, and Figures 8 and 9. Our results also match findings by Avula B, et al. [22]. However, when the results obtained for the biological effects of both extracts were compared for radical scavenging of ABTS•+ radical, the Palestinian sage extract showed higher inhibitory oxidation activity: the test showed IC50=309 mg/mL whereas the Saudi Sage extract exerted lower antioxidant capacity and the in vitro test showed IC50=790.7 mg/mL. Data are reported in Figure 10. Similar free radical scavenging values were reported using a DPPH test by Khiya Z, et al. [23], in Moroccan essential oils of Salvia officinalis L. by maceration in a hydroalcoholic mixture (Methanol/Water: 70/30). The IC50 shown in that study was 309.42 mg/mL.
| No. | Identifications | Rt [min] |
|---|---|---|
| 1 | L-camphor | 13.06 |
| 2 | Camphor oxime | 14.18 |
| 3 | Icosapentaenoic acid | 14.57 |
| 4 | Timnodonic acid | 14.85 |
| 5 | Glycerol | 15.33 |
| 6 | β-Eudesmol, TMS | 16.25 |
| 7 | Thymol, TMS | 16.55 |
| 8 | Butanedioic acid, 2TMS | 16.59 |
| 9 | (S)-(-)-α-Terpineol | 16.88 |
| 10 | α-Terpinyl acetate | 17.72 |
| 11 | Caryophyllene | 19.46 |
| 12 | Aromandendrene | 19.88 |
| 13 | Humulene | 20.27 |
| 14 | L-Threitol 4TMS | 20.43 |
| 15 | Tyrosol 2TMS | 22.18 |
| 16 | Caryophyllene oxide | 23.03 |
| 17 | L-(-)-Arabitol, 5TMS | 24.46 |
| 18 | D-(-)-Tagatose, pentakis(trimethylsilyl) ether, methyloxime (anti) | 27.15 |
| 19 | D-(-)-Tagatose, pentakis(trimethylsilyl) ether, methyloxime (syn) | 27.33 |
| 20 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1E)- | 27.59 |
| 21 | d-Mannose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1E)- | 27.93 |
| 22 | D-Mannitol, 6TMS | 28.05 |
| 23 | Palmitic Acid TMS | 30.59 |
| 24 | Myo-Inositol 6TMS | 30.73 |
| 25 | Phytol TMS | 32.44 |
| 26 | Stearic acid TMS | 33.55 |
| 27 | Ferruginol, trimethylsilyl ether | 33.81 |
| 28 | PROPOSED COMPOUND [2-Methoxyestrone, TMS] | 33.88 |
| 29 | Kolavelool acetate | 35.17 |
| 30 | PROPOSED COMPOUND [Dihydrocodeine] | 36.88 |
| 31 | Trimethylsilyl 7-isopropyl-1,1-dimethyl-5,6-bis((trimethylsilyl)oxy)-1,2,3,4,4a,9,10,10a-octahydrophenanthrene-4a-carboxylate [MAJOR PEAK MATCH 670] | 37.79 |
| 32 | Ursolic acid | 48.95 |
| 33 | Ursolic acid isomer | 49.58 |
Numerous studies [24-28] have indicated the importance of natural compounds and their biological impacts on viruses, in this study, the effectiveness of phytochemical compounds has been screened for antiviral capabilities by investigating their ability to inhibit HIV through inhibition of HIV
| NO. | ID | Formula | RT | [M+H]+ (m/z) |
|---|---|---|---|---|
| 1 | Choline | C5 H13 N O | 0.60 | 104.10 |
| 2 | trans-3-Indoleacrylic acid | C11 H9 NO2 | 2.05 | 188.07 |
| 3 | 6-Pentyl-2H-pyran-2-one | C10 H14 O2 | 3.99 | 167.10 |
| 4 | Miquelianin | C21 H18 O13 | 4.22 | 479.08 |
| 5 | (E)-2-decenoicacid | C10 H18 O2 | 4.54 | 171.13 |
| 6 | Heptanophenone | C13 H18 O | 5.00 | 191.14 |
| 7 | Napyradiomycin D | C24 H24 Cl2 O5 | 5.18 | 463.10 |
| 8 | Nepetin | C16 H12 O7 | 5.81 | 317.06 |
| 9 | Oxybutynin | C22 H31 N O3 | 6.31 | 358.23 |
| 10 | 9S,13R-12-Oxophytodienoic acid | C18 H28 O3 | 6.49 | 293.21 |
| 11 | Scrophulein | C17 H14 O6 | 7.03 | 315.08 |
| 12 | 2-Methoxyestrone | C19 H24 O3 | 7.23 | 301.17 |
| 13 | Estriol | C18 H24 O3 | 7.79 | 289.17 |
| 14 | 2-[(3,3-dimethyl-2-oxobutyl) thio]-6-oxo-4-pyridin-3-yl-1,6-dihydropyrimidine-5-carbonitrile | C16 H16 N4 O2S | 8.34 | 329.11 |
| 15 | Caryophyllose | C12 H24 O8 | 8.57 | 297.15 |
| 16 | NP-006105 | C15 H16 O5 | 8.96 | 299.09 |
| 17 | Desotamide E | C34 H50 N8 O7 | 9.16 | 683.38 |
| 18 | 1-Linoleoylglycerophosphocholine | C26 H50 N O7 P | 9.75 | 520.33 |
| 19 | (-)-spirocochlealactone A | C42 H50 O8 | 9.86 | 683.35 |
| 20 | 1-(5-Fluoro-2-pyridinyl)-N-(4-isopropylbenzyl)-2-methyl-1-propanamine | C19 H25 F N2 | 10.37 | 301.20 |
| 21 | Oleanolic acid | C30 H48 O3 | 11.47 | 439.35 |
| 22 | 16-deethylindanomycin methyl ester | C30 H41 N O4 | 11.58 | 480.31 |
| 23 | Chivosazole F | C41 H57 N O8 | 12.56 | 692.41 |
| 24 | 2,2'-Methylenebis[4-methyl-6-(1-methylcyclohexyl) phenol] | C29 H40 O2 | 13.08 | 421.31 |
| 25 | IN00350 | C39 H54 F N3 Ot | 13.97 | 632.41 |
| NO. | ID | Formula | RT | [M+H]+ (m/z) |
|---|---|---|---|---|
| 1 | Choline | C5 H13 N O | .59 | 104.10 |
| 2 | Betaine | C5 H11 N O2 | .64 | 118.08 |
| 3 | Pantothenic acid (Vitamin B5) | C9 H17 N O5 | 2.09 | 220.11 |
| 4 | trans-3-Indoleacrylic acid | C11 H9 NO2 | 2.06 | 188.07 |
| 5 | Tuliposide B | C11 H18 O9 | 3.90 | 295.10 |
| 6 | NP-021018 | C12 H18 O4 | 3.98 | 227.12 |
| 7 | 1-Phenyl-2-butanone | C10 H12 O | 4.22 | 149.09 |
| 8 | (E)-2-decenoicacid | C10H18 O2 | 4.54 | 171.13 |
| 9 | 2,4-Dimethyl-1-vinylbenzene | C10 H12 | 5.00 | 133.10 |
| 10 | (8S)-N-(2-Amino-2-oxoethyl)-7-[(1-methylcyclopropyl)carbonyl]-3-(3-nitrophenyl)-1-oxa-2,7-diazaspiro[4.4]non-2-ene-8-carboxamide | C20 H 23 N5 O6 | 5.81 | 430.17 |
| 11 | 5,7-dihydroxy-3-(4-hydroxyphenyl)-6-methoxy-4H-chromen-4-one | C 16 H12 O6 | 6.31 | 301.07 |
| 12 | Valdecoxib | C16H14 N2 O3 S | 7.03 | 315.07 |
| 13 | NP-000465 | C17 H14O6 | 7.23 | 315.08 |
| 14 | Piperalin | C16 H21 Cl2 N O2 | 8.35 | 330.09 |
| 15 | 5α-Androstan-3,6,17-trione | C19 H26 O3 | 8.95 | 303.19 |
| 16 | 1,4-Androstadiene-3,17-dione | C19 H24 O2 | 9.16 | 285.18 |
| 17 | (-)-spirocochlealactone A | C42 H 50 O8 | 9.84 | 683.35 |
| 18 | Anthocidin B | C21 H33 NO4 | 10.34 | 364.24 |
| 19 | 16-deethylindanomycin methyl ester | C30 H41 N O4 | 11.58 | 480.30 |
| 20 | 3-[(3S,4S,21R)-14-Ethyl-21-(methoxycarbonyl)-4,8,13,18-tetramethyl-20-oxo-9-vinyl-3-phorbinyl]propanoic acid | C35 H36 N4 O5 | 12.35 | 593.27 |
| 21 | 3-(3-Methyl-3H-diazirin-3-yl)-propamino-carbonyl-Ne-L-lysine | C12 H23 N5 O3 | 13.09 | 286.18 |
| 22 | N-Isobutyl-1-{3-[2-(tetrahydro-2H-pyran-4-ylamino)-4-pyridinyl]-2,6-naphthyridin-1-yl}-4-piperidinecarboxamide | C28 H36 N6 O2 | 13.29 | 489.29 |
| No. | ID | Formula | RT [min] |
|---|---|---|---|
| 1 | Scrophulein | C17 H14O6 | 7.03 |
| 2 | Hispidulin | C17 H14O6 | 6.31 |
| 3 | Nepetin | C16 H12 O7 | 5.81 |
| 4 | Glycitein | C16 H12 O5 | 7.63 |
| 5 | Apigenin | C15 H10 O5 | 6.21 |
| 6 | Luteolin | C15 H10 O6 | 5.75 |
| 7 | Kaempferol 7- (6"-p-succinylglucoside) | C25 H24 O14 | 5.49 |
| 8 | Neodiosmin | C28 H32 O15 | 5.10 |
| 9 | Isorhamnetin 3-glucuronide | C22 H20 O13 | 4.78 |
| 10 | Apigenin 7-O-glucuronide | C21 H18 O11 | 5.01 |
| 11 | Apigetrin | C21 H20 O10 | 5.06 |
| 12 | Isokaempferide | C16 H12 O6 | 6.55 |
| 13 | Rhoifolin | C27 H30 O14 | 4.94 |
| 14 | Kuromanin | C21 H20 O11 | 4.67 |
| 15 | Scutellarein 7-glucuronide | C21 H18 O12 | 5.17 |
| 16 | Apigenin 7- (6"-malonylglucoside) | C24 H22 O13 | 5.44 |
| NO. | ID | Formula | RT [min] |
|---|---|---|---|
| 1 | Scrophulein | C17 H14O6 | 7.03 |
| 2 | Glycitein | C16 H12 O5 | 7.63 |
| 3 | Apigenin | C15 H10 O5 | 6.22 |
| 4 | Luteolin | C15 H10 O6 | 5.75 |
| 5 | Nepetin | C16 H12 O7 | 5.82 |
| 6 | Kaempferol 7- (6"-p-succinylglucoside) | C25 H24 O14 | 5.50 |
| 7 | Apigenin 7-O-glucuronide | C21 H20 O11 | 5.02 |
| 8 | Rhoifolin | C27 H30 O14 | 4.95 |
| 9 | Hispidulin | C17 H14O6 | 5.19 |
| 10 | Apigetrin | C21 H20 O10 | 5.07 |
| 11 | Isorhamnetin 3-glucuronide | C22 H20 O13 | 4.79 |
Figure 8. Structures of 16 flavonoids detected by UHPLC in Saudi sage.
showed approximately) 25.1 ± 10.6) μg/mL. In contrast, the inhibitor control pepstatin A showed significant inhibition with (28.5 ± 22.4) μg/mL more than polar extracts, while the IC50 of Saudi sage extracts was =5.1 mg/mL and IC50 in Palestinian sage extracts was = 6.7 mg/mL which indicates that neither of them significantly inhibited HIV-1 PR. Supplemental in-depth studies are needed, to illustrate the biological function and mechanism of action of active extracts and to define their active constituents by polar fractionation with DCM/ methanol. Each faction should then be studied separately with other microorganisms
Figure 9. Structures of 11 flavonoids detected by UHPLC in Palestinian sage.
Salvia officinalis L. (sage) is an important aromatic and medicinal plant, due to its bioactive components, primary and secondary metabolites. Our findings show that Palestinian sage extracts exerted greater antioxidant activity than Saudi extracts the test showed IC50=309 mg/mL comparing to IC50=790.7 mg/mL of Saudi extracts, although there was less diversity in flavonoid compounds in Palestinian extracts in methanol/DCM solvents that reported 11 flavonoid compounds comparing to the Saudi extracts that reported 16 flavonoid compounds, which indicates that other chemical compounds also contributed to this biological activity. These compounds make sage a plant of interest and potential application in pharmaceutical and medical settings. Moreover, natural products may be considered the best and safest source of antioxidants, thereby providing new perspectives from which to explore more of these compounds.
We would like to thank Dr. Najeh Kharbatia, Salim Sioud (Core Labs, KAUST, Thuwal, Saudi Arabia) and Dr. Inas Al-Younis for aiding in technical support and data analysis. The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number (MoE-IF-20-02/12). MJ and A-HE would like to thank KAUST for financial support.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Journal of Pharmacognosy & Natural Products received 606 citations as per Google Scholar report