Full Length Research Paper - (2022) Volume 9, Issue 10
Received: 14-Sep-2022, Manuscript No. jreac-22-74705;
Editor assigned: 16-Sep-2022, Pre QC No. P-74705;
Reviewed: 29-Sep-2022, QC No. Q-74705;
Revised: 14-Oct-2022, Manuscript No. R-74705;
Published:
21-Oct-2022
, DOI: 10.37421/2380-2391.2022.9.392
Citation: Khan, Rizwan Ullah, Muhammad Hamayun, Ataf Ali Altaf and Samia Kausar, et al. “Toxic Heavy Metals Assessment and Removal of Iron Ions from the Industrial Wastewater of Rawalpindi (Pakistan).” J Environ Anal Chem 9 (2022): 392.
Copyright: © 2022 Khan RU, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
The heavy metals like iron, arsenic, cadmium, chromium, copper, lead, manganese, mercury, nickel, silver, and zinc in industrial wastewater pose threats to public health. The heavy metals toxicity causeS the damage of numerous human organs and also causing diseases in the human body. Iron is a heavy metal and it discharge into water from industrial wastes, natural geological sources, domestic wastes and various byproducts. Excess quantity of iron causes speedy rise in pulse rate, clotting of blood in blood vessels, drowsiness, and hypertension. In the current study assessment of toxic heavy metals carried out for wastewater, collected from small industrial estate (Taxila), Rawalpindi in first phase. In the second phase the iron ions removal carried out by using a novel biosorbent obtained from the seeds of Monotheca buxifolia (M. buxifolia) plant. The analysis results of wastewater show that iron is found in above allowable limit as per National Environmental Quality Standards (NEQS) for municipal and liquid industrial effluents, set by the government of Pakistan. The analysis results under different conditions of pH, contact time, and temperature in batch sorption process indicates that high metal ions of iron concentration reduced to permissible limit of industrial effluent NEQS (Pakistan) at the optimal conditions of pH 10 at 50°C temperature in 60 minutes contact time by using biosorbent of M. buxifolia . Biosorbent of M. buxifolia eliminated 99.91% iron (III) ions from industrial wastewater, collected from Rawalpindi industrial area. In the light of analysis result the novel biosorbent obtained from M. buxifolia is a low-cost, easily available, and environmental friendly biosorbent for the removal of iron from industrial wastewater.
Heavy metals • Industrial wastewater • Toxicity • Monotheca buxifolia • NEQS
Among all the valuable resources, water is absolutely important for life and all living things need water for their existence. Human beings can live for a long time without food but cannot live without water [1]. Human utilize fresh water for various purposes. The three main broad uses for which people draw water from natural water bodies are industrial activities, irrigation of crops, and domestic activities [2]. Industrial wastewaters have diverse configurations dependent on the nature of industry and materials treated. Among these wastewaters some are easily decomposable, organically very durable, mostly inorganic and potentially inhibitory [3]. The untreated industrial water contamination has many harmful effects on ecosystem and causes different kinds of diseases in human beings and animals [4]. Industrial wastewater can be polluted with a vast diversity of toxic chemicals, with heavy metals, and organics compounds. Unlike organic toxic compounds, inorganic heavy metals are non-biodegradable [5]. The heavy metals like arsenic, cadmium, chromium, copper, lead, manganese, mercury, nickel, and zinc pose threats to public health because these are insistent in nature and have increase affinity in biological systems [6]. Similarly a number of heavy metals like manganese and iron are important for some physiological and biochemical activities in human body, high concentration in the body can put excited health effects. The toxicity of some heavy metals could be acute while some others could be chronic after exposure to long time. This acute and chronic toxicity cause to damage of numerous organs in the body like the liver, kidney, brain, and lungs, causing diseases in the human body [7]. Although a number of techniques can be used for the treatment of wastewater loaded with heavy metals, it is essential to note that the selection of the best appropriate treatment for metal polluted wastewater depends on some elementary parameters like pH, contact time, initial metal concentration, quantity of adsorbent, environmental effect, the complete treatment efficiency comparison with other techniques, as well as economic factors like operational charges and capital investment [8]. Biosorption technique have some advantages on other techniques like eco-friendly, low-cost, reuse, high efficiency, and possibility of metal recovery. Usually, the used agricultural residues and food waste are preferred because they cover three main structural components comprising lignin, hemicelluloses and cellulose [9].
The iron compounds play a central role in wastewater, in limnology, and in water supplies. Iron occurs in solution either in ferrous (Fe2+) or ferric (Fe3+) ionic state. The ferrous iron is oxidized to ferric iron [10]. The industries causes iron contaminations are pipe making, steel manufacturing, and civil engineering [11]. Iron causes staining in clothes and conveys a bitter flavor. It discharges into water from industrial wastes, natural geological sources, domestic wastes and various byproducts. Excess quantity of iron causes speedy rise in pulse rate, clotting of blood in blood vessels, drowsiness and hypertension [12].
In the current study assessment of toxic heavy metal ions carried out for wastewater, collected from small industrial estate (Taxila), Rawalpindi in first phase. In the second phase the iron ions removal carried out by using a novel biosorbet obtained from the seeds of Monotheca buxifolia (M. buxifolia) plant, locally known as “Gurgura” in Pakistan.
Study area
Rawalpindi District location is in Potohar plateau in the north of the Punjab province. It lies between 33° 04 ’ to 34° 01’ in north latitudes and 72° 38’ to 73° 37’ in east longitudes. This city keeps 4.5 million populations and the total area of the district is 5,286 km2 with a density of 845 persons /km2. The district is divided into seven tehsils i.e., Rawalpindi, Murree, Gujjar Khan, Kotli Sattian, Kahuta, Kallar Seddan, and Taxila [13]. Taxila is a significant archaeological area in the Rawalpindi district of the Punjab region in Pakistan. It is located approximately 33 km northwest of Rawalpindi [14]. The city of Taxila is surrounded by several famous industrial sectors i.e., Pakistan ordinance factories, heavy mechanical complex, Hattar industrial estates & air weapon complex. The weather of the Taxila city is soggy subtropical with usual rainfall of 990 mm [15]. Taxila is a semi urban industrial estate of 118 acres, established in 1994 at a distance of 7 km at Bahatar road [16]. The industries like marble & granite, papers, pharmaceuticals, chees & dairy, handicraft, iron, engineering equipment, and flour mills are functioning in Taxila small industrial estate. These industries discharge their wastewater in a drain. The local people use the drain water for irrigation purpose and this water polluting the groundwater reservoirs. The map of Rawalpindi and sample collection area (Small Industrial Estate Taxila) is given in Figure 1.
Monotheca buxifolia
M. buxifolia (Figure 2), a member of genus Monotheca, belongs to family Sapotaceae, is a thorny, ever green, wild, and medicinal plant from 7-10 meter in height was selected for the research work. Fruit of M. buxifolia were collected from the hilly area of Shakardara, a town of district Kohat (Khyber Pakhtunkhwa) province of Pakistan. M. buxifolia seeds coat were utilized as a novel biosorbent for the removal iron ions in this research work (Figure 2).
Samples collection
Total 02 samples of wastewater collected from the small industrial estate Taxila (Rawalpindi), in polyethylene canes. One sample collected from the main drain carrying wastewater from various industries and the other sample collected from the outlet of the main drain, irrigating agricultural land as shown in Figure 3.
Samples preservation
After the samples collection the samples immediately filtered and preserved by the addition HNO3 (pH>2) to stop the metals from precipitation and complex formation as per standard method [17]. Tag each sample and properly sealed for further analysis in standard laboratory. The samples analyzed as soon as possible after collection.
Stock solution preparation
Dissolved 40 g NaOH (Merck) in 1000 ml deionized water for the preparation of 1M basic solution in a volumetric flask. Added 63 ml of HNO3 (Duksan) in a volumetric flask and raised the volume up 1000 ml through deionize water for the preparation of 1M acid solution. The required solutions 0.1M HNO3/NaOH were prepared from stock solutions for the adjustment of sample pH during pH study [18]. Different concentration of standard solution of different metals prepared from standards calibration stock metal solutions during heavy metals ions analysis through Atomic Absorption Spectrophotometer (ASS).
Chemicals and reagents
All the chemicals used in this research work were of analytical grade. The NaOH (Merck) and HNO3 (Duksan) were used for stock solution preparation with deionized water to adjust the solution pH. Solutions of different concentration were prepared from stock standard solution for calibration of AAS during heavy metals ions analysis. Distil water was used thoroughly in the experimental work. Dried air, acetylene, and nitrogen gas cylinder was use in AAS for metal ions determination.
Glassware and miscellaneous laboratory materials
Glass Sample Bottles, Beakers, Pipettes, Volumetric Flasks with Stopper, Graduated Cylinders, Funnel, Glass Rod, Pipette Filler, Whatman Filter Papers-44, Polyethylene Canes, Masking Tape, Calculators, Digital Camera, Watch, Wash Bottles, Scissor, Safety Goggles, Crucible, Crucible Tongs, Erlenmeyer Flasks, Pen, Note Book, Permanent Marker etc.
Apparatus
The following major apparatus were used in research study:
1. Atomic Absorption Spectrophotometer (AAS 700) and (GBC 932 Plus) utilized for heavy metals estimation in wastewater.
2. Usage of Orbital Shaker (KJ -201BD) for iron adsorption studies.
3. pH measurement carried out by using pH Meter (OHAUS ST 10).
4. Hot Plate Stirrer (SCILOGEX MS-H280-Pro) used for iron adsorption studies.
5. Analytical Balance (Sartorius CP323P) utilized for weight measurement.
Wastewater analysis for heavy metal ions detection
The industrial wastewater samples, collected from small industrial estate (Rawalpindi) of Punjab province were analysed for toxic heavy metal ions i.e., arsenic, cadmium, chromium, copper, iron, lead, manganese, mercury, nickel, silver and zinc by using atomic absorption spectrophotometers.
Determination of arsenic
The industrial wastewater samples analysis carried out for arsenic ions determination by using 3114 C. Continuous Hydride Generation/Atomic Absorption Spectrometric Method in laboratory [19]. In this procedure set all the operational parameters of the double beam AAS (GBC 932 Plus), controlled by a software. Then connected the instrument with furnace assembly and electrical requirement system. Set the nitrogen supply regulator at 140 kPa. Ensured the supply of nitrous oxide- acetylene flame system. After that, connected the hydride generator continuous flow system with autos ampler. When set up completed, turned on the system. Now calibrated the instrument with standardized arsenic solution. Used sample delivery system for injection of samples and automated analysis of arsenic carried out. Report printed after completion of analysis.
Determination of mercury
The industrial wastewater samples analysis carried out for mercury ions determination by using 3112 B. Cold-Vapor Atomic Absorption Spectrometric Method in laboratory [19]. In this procedure, set all the operational parameters of the double beam AAS (GBC 932 Plus), controlled by a software. Connected the mercury vapour generation accessory to the instrument. Turned on the system and calibrated the instrument with standardized mercury solution. Then transferred the sample in a reaction flask. Finally, determined the peak height of sample from recorder chart and read mercury value from standard curve set. Report printed after completion of analysis.
Determination of cadmium, chromium, copper, iron, lead, manganese, nickel, silver, and zinc
The industrial wastewater samples analysis carried out for cadmium, chromium, copper, iron, lead, manganese, nickel, silver, and zinc ions determination by using 3111 B. Direct Air- Acetylene Flame Method in laboratory [19]. In this method installed a hollow-cathode lamp for the desired metal in the AAS 700 and set the wavelength for desired metal. After that set slit width for the element being measured. Turn on instrument and warm up from 10 to 20 minutes, till energy source stabilized. Then installed appropriate burner head and adjusted burner head position. Turn on air and acetylene and adjusted flow rate to give maximum sensitivity for the metal being measured. Zero the instrument and aspirated a standard solution and adjusted aspiration rate of the nebulizer to get maximum sensitivity. Aspirated the sample and determined its absorbance. Finally calculated the concentration of each metal ion, in mg/l and report printed after completion of analysis.
The atomic absorption spectrophotometer AAS 700 (A) and GBC 932 Plus (B) are shown in Figure 4.
Adsorption process
Batch adsorption experiments were performed for the removal of iron concentration by using M. boxifolia as biusorbent in pH, time contact and temperature studies.
pH Study: pH study was performed in the pH range of 2-10, by taking 50 ml of industrial wastewater in 100 ml Erlenmeyer flasks. The pH of the flasks was adjusted, using pH meter with the help of 0.1M HNO3 and 0.1M NaOH solution. The pH study was conducted at room temperature. Each flask was furnished with 0.1 g of biosorbent and the suspension of different flasks was shaken on orbital shaker for 12 hours at 110 rpm. The suspension was filtered through whatman filter paper and the final concentration of the iron ions were determined by using AAS.
Contact time study: In contact time study, added 50ml of industrial wastewater from samples in 100 ml Erlenmeyer flasks. Adjusted the optimum pH of the samples through pH meter by using 0.1M NaOH and 0.1M HNO3 solution. Then added 0.1 g of biosorbent to each flask and tag the flasks and placed all the 6 flasks in a sequence on orbital shaker. Fixed the 110 rpm of orbital shaker and shake all the samples for 5, 10, 20, 30, 40, and 60 minutes at room temperature. In final stage, filtered the solution of each flask after the desired time through filter paper whatman No. 44 and detected the concentration of iron ions by using AAS.
Temperature study: In temperature study, Added 50ml of industrial wastewater from samples in 100 ml Erlenmeyer flasks. Adjusted the optimum pH of the samples through pH meter by using 0.1M NaOH and 0.1M HNO3 solution. Added 0.1 g of biosorbent to each flask. Then tag the flasks and place all the flasks in a sequence on hot plate stirrer. Fixed the 110 rpm of hot plate stirrer and agitated all the samples for optimum time (1 h) at a temperature of 25, 30, 35, 40, 45, and 50°C. Finally, filtered the solution of each flask after the desired temperature through Whatman No. 44 filter paper and detected the concentration of iron ions by using AAS.
The orbital shaker KJ -201BD (A) and hot plate stirrer SCILOGEX MSH280- Pro (B) are shown in Figure 5, used in adsorption process.
Wastewater analysis for iron ions detection
The novel biosorbent used for the elimination of iron, existing in high concentration in industrial wastewater of Taxila (Rawalpindi) during batch adsorption process. The final concentration of iron after the adsorption process was detected by using AAS (AAS 700).
Biosorbent efficiency calculation
Biosorbent M. buxifolia efficiency was calculated by the following relation:
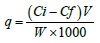
Where, q is the quantity of adsorbent absorbed (mg/gm), Ci is initial, Cf is final concentrations (mg/l) and W is the weight of adsorbent (gm).
Percent metal ion removal (%MR) was calculated by using the equation:
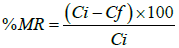
Where, Ci is initial concentrations (mg/l) and Ce is equilibrium concentrations (mg/l).
Determination of heavy metals ions
Table 1 indicated the heavy metal ions concentration, existing in wastewater samples, collected from small industrial estate Taxila (Rawalpindi) (Figure 6).
| Metal | Concentration (mg/l) in Sample-1 | Concentration (mg/l) in Sample-2 | NEQS for Municipal and Liquid Industrial Effluents |
|---|---|---|---|
| Arsenic | 0.00 | 0.00 | 1.0 |
| Cadmium | 0.02 | 0.02 | 0.1 |
| Chromium | 0.03 | 0.04 | 1.0 |
| Copper | 0.18 | 0.19 | 1.0 |
| Iron | 44.58 | 34.12 | 8.0 |
| Lead | 0.00 | 0.00 | 0.5 |
| Manganese | 0.43 | 0.47 | 1.5 |
| Mercury | 0.00 | 0.00 | 0.01 |
| Nickel | 0.08 | 0.14 | 1.0 |
| Silver | 0.12 | 0.10 | 1.0 |
| Zinc | 0.20 | 0.13 | 5.0 |
As the results shows, arsenic, lead, and mercury ions were not found in Taxila wastewater. Cadmium level was observed 0.02 mg/l in both samples. Chromium, copper, manganese, and nickel were ranged from 0.03-0.04 mg/l, 0.18-0.19 mg/l, 0.43-0.47 mg/l, and 0.08-0.14 mg/l in sample-1 and sample-2 respectively. While the concentration of silver, and zinc ranged from 0.10-0.12 mg/l and 0.13-0.20 mg/l in sample-2 and smple-1 respectively. All these toxic heavy metal ions were found below permissible limit of liquid industrial effluents NEQS of Pakistn (Table 1). The high content of iron ions were observed in industrial wastewater samples, taken from Taxila (Rawalpindi) in a ranged from 44.58 to 34.12mg/l, above from the permissible value of liquid industrial effluents NEQS of Pakistan (Table 1). The industries of iron and engineering works operating in the study area may be the cause of high iron content in wastewater. Iron in drinking water at higher concentrations may cause serious health problems like aching neck, severe constipation, problems in hips and arms, gastric ulcer, head ache, muscle cramps, rapid pulse, diarrhea, loss of weight and appetite, apathy, and lethargy [20]. The local population can suffer from all these health problems due to seepage in soil and mixing of industrial wastewater from drains into the drinking water sources. Therefor keeping in view the toxicity of iron in high concentration, biosorption technique was applied for the removal of iron ions from industrial wastewater.
Biosorption parameters effects
Effect of pH on iron ions removal: The Table 2 given below illustrated the effect of pH on iron removal from wastewater samples collected from industrial estate Taxila, Rawalpindi.
| pH | Concentration (mg/l) | Adsorbance (mg/g) | Removal of ions (%) | Initial concentration (mg/l) |
|---|---|---|---|---|
| 2 | 18.01 | 13.28 | 59.60 | Sample-1 44.58 |
| 4 | 14.38 | 15.10 | 67.74 | |
| 6 | 0.73 | 21.92 | 98.36 | |
| 8 | 0.24 | 22.17 | 99.46 | |
| 10 | 0.17 | 22.20 | 99.61 | |
| 2 | 28.19 | 2.96 | 17.37 | Sample-2 34.12 |
| 4 | 16.28 | 8.92 | 52.28 | |
| 6 | 0.97 | 16.57 | 97.15 | |
| 8 | 0.69 | 16.71 | 97.97 | |
| 10 | 0.69 | 16.71 | 97.97 |
Graphically presentation of the iron ions sorption and percentage removal from Rawalpindi industrial wastewater samples at different pH is given in Figures 7 and 8 respectively.
The batch adsorption experiment results of this study evidently specified that the biosorbance rate and percentage removal of iron ions by biosorbent slowly increase in the initial stage of the process from pH 2-4 for sample-1, collected from Taxila (Rawalpindi) industrial estate wastewater. The adsorption rate was found 13.28 mg/g and percentage removal was only 59.60 at pH 2 and it was found 15.10 mg/g iron ions adsorption and 67.74% removal of iron ions at pH 4. When the pH further increases the adsorption rate increases and thus the maximum percentage removal obtained. The concentration of iron ions plummeted to permissible limit from 44.58 mg/l to acceptable limit i.e., 0.17 mg/l at pH 10 and thus eliminated 99.61% iron ions by using biosorbent of M. buxifolia. Similarly the biosorption rate and percentage removal of iron ions by biosorbent was very slow in the initial stage of the process from pH 2-4 for sample-2. When the pH increased from 4-10, the uptake of iron ions and percentage removal increased by bisorbent. The maximum percentage removal of iron ions were observed 97.97 and the biosorbent brought the concentration of iron ion from 34.12 mg/l to the allowable limit i.e., 0.69 mg/l.
At low pH values, the adsorbent surface surrounded by hydronium ions, which reduced the metal ion contact with binding sites of biosorbent by greater repulsive forces and thus lower adsorption takes place. On the other hand, as the pH was increased, the opposing effect of hydrogen ions decreased and more ligands were accessible. Therefore, at high pH values, the complete surface on the biosorbent became more negative and adsorption increased [21]. It is most possible that at low pH values, due to columbic repulsion positively charged, surface will not favor the binding with iron ions. When the pH values increase, the surface becomes more and more negatively charged and therefore favoring iron ion binding [22].
Effect of time contact on iron ions removal: Biosorbent produced from M. buxifolia found a best biosorbent for the removal of iron ions from wastewater Taxila (Rawalpindi) in batch adsorption process as complete iron ions removal observed up to 100% at optimum pH in 60 minutes in different intervals of time. The concentration of iron ions dropped from 44.58 mg/l to 0.00 mg/l in sample-1 wastewater and dropped from 34.12 mg/l to 0.00 mg/l in sample-2 wastewater.
The removal of iron ions from wastewater samples of Taxila (Rawalpindi) as a function of different contact time intervals are represented in Table 3.
| pH | Concentration (mg/l) | Adsorbance (mg/g) | Removal of Ions (%) | Initial Concentration (mg/l) |
|---|---|---|---|---|
| 5 | 0.02 | 22.28 | 99.95 | Sample-1 44.58 |
| 10 | 0.01 | 22.28 | 99.97 | |
| 20 | 0.00 | 22.29 | 100 | |
| 30 | 0.00 | 22.29 | 100 | |
| 40 | 0.00 | 22.29 | 100 | |
| 60 | 0.00 | 22.29 | 100 | |
| 5 | 0.04 | 17.04 | 99.88 | Sample-2 34.12 |
| 10 | 0.03 | 17.04 | 99.91 | |
| 20 | 0.03 | 17.04 | 99.91 | |
| 30 | 0.02 | 17.05 | 99.94 | |
| 40 | 0.01 | 17.05 | 99.97 | |
| 60 | 0.00 | 17.06 | 100 | - |
The association between contact time with rate of adsorbtion and percentage elimination of iron ions from industrial wastewater with M. buxifolia biosorbent is shown in Figures 9 and 10 respectively.
The result shows that the removal efficiency of iron ions by the biosorbent increased initially very rapidly with contact time in 10 minutes. After that the adsorption rate and removal efficiency remained constant until equilibrium was reached after stirring for 60 minutes at optimum pH 10 at room temperature. 100% removal of iron ions were achieved at 22.29 mg/g adsorption rate and the concentration of ions dropped from 44.58 mg/l to 0 mg/l in 1 hour for sample-1. While for sample-2, the removal efficiency of iron ions by the biosorbent increased gradually till equilibrium was reached in the same rate in 60 minutes stirring at optimum pH 10 at room temperature. 100% removal of iron ions were achieved at 17.06 mg/g adsorption rate and the concentration of ions dropped from 34.12 mg/l to 0 mg/l in 1 hour. The constant value at equilibrium period is due to the empty space is completely filled by heavy metal ions and with the passage of time, there is no available sites for heavy metal ions binding to the cell wall surface. As a result, a repulsive force existed [23].
Effect of temperature on iron ions removal: The effect of temperature on iron ions removal from industrial wastewater samples collected from Taxila (Rawalpindi) industrial estate have been summarized in Table 4 and sketchily shown in Figures 11 and 12 respectively.
| Temperature (°C) | Concentration (mg/l) | Adsorbance (mg/g) | Removal of Ions (%) | Initial Concentration (mg/l) |
|---|---|---|---|---|
| 25 | 0.07 | 22.25 | 99.84 | Sample-1 44.58 |
| 30 | 0.05 | 22.26 | 99.88 | |
| 35 | 0.05 | 22.26 | 99.88 | |
| 40 | 0.04 | 22.27 | 99.91 | |
| 45 | 0.04 | 22.27 | 99.91 | |
| 50 | 0.04 | 22.27 | 99.91 | |
| 25 | 0.28 | 16.92 | 99.17 | Sample-2 34.12 |
| 30 | 0.10 | 17.01 | 99.70 | |
| 35 | 0.08 | 17.02 | 99.76 | |
| 40 | 0.07 | 17.02 | 99.79 | |
| 45 | 0.06 | 17.03 | 99.82 | |
| 50 | 0.06 | 17.03 | 99.82 |
The above results expressed that the up taken efficiency of iron ions by the biosorbent increased initially very fast with increase in temperature at 25°C by optimum pH 10 and optimum contact time (60 minutes). The sorbent rate was found 22.25 mg/g and 99.84% iron ions were removed at this stage. After that the adsorption rate and removal efficiency continued at a constant speed up as the equilibrium reached after stirring for optimum 1 hour time at optimum pH 10. Finally, 99.91% removal of iron ions were obtained at 22.27 mg/g adsorption rate at a temperature of 50°C and the concentration of ions decreased from 44.58 mg/l to 0.04 mg/l in 1 hour for sample-1. The elimination efficiency of iron ions changed at a slow rate from 30-50°C. In case of sample-2, the percentage removal efficiency of iron ions by the biosorbent increased initially and was observed 99.17 at the sorbent rate of 16.92 mg/g at 25°C, until equilibrium established at optimum contact time 60 minutes and optimum pH 10. Finally the concentration of iron ions were dropped from 34.12 mg/g to 0.06 mg/g at 17.03 mg/g adsorption rate and 99.82% iron ions were removed at 50°C. The elimination efficiency of iron ions changed at a slow rate from 30-50°C. The removal and sorption rate of iron ions enhanced with increase in temperature. The sorption rate might have increased because of the higher diffusion rates and better movement of ions at high temperatures [24]. Further, increasing temperature increases the kinetic energy and surface activity adsorbate [25].
The analysis results revealed that iron is found in above allowable limit as per NEQS for municipal and liquid industrial effluents set by the government of Pakistan in samples collected from Taxila, Rawalpindi. M. buxifolia wild plant seeds obtained and then utilized a cheap, environmental friendly and easily available novel biosorbent for the removal of iron (III) ions existing in high concentration in wastewater of study industrial areas. The analysis results of these parameters under the different parameters conditions in batch sorption process indicates that high metal ions of iron concentration reduced to permissible standards of industrial effluent NEQS (Pakistan) at the optimal conditions of pH 10 at 50°C temperature in 60 minutes contact time. Biosorbent of M. buxifolia eliminated maximum 99.91% iron (III) ions from industrial wastewater, collected from Taxila (Rawalpindi) industrial area. The novel biosorbent can be used for the removal and reduction of other heavy metals from industrial wastewater, polluting our valuable water sources.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Journal of Environmental Analytical Chemistry received 1781 citations as per Google Scholar report