Research Article - (2023) Volume 13, Issue 2
Received: 02-Jan-2023, Manuscript No. JBPBT-23-85238;
Editor assigned: 05-Jan-2023, Pre QC No. JBPBT-23-85238 (PQ);
Reviewed: 20-Jan-2023, QC No. JBPBT-23-85238;
Revised: 03-Mar-2023, Manuscript No. JBPBT-23-85238 (R);
Published:
10-Mar-2023
, DOI: 10.37421/2155-9821.2023.13.565
Citation: Tarse, Dagne Tarle and Shimelis Admasu Emire.
"The State of the Art of PH Sensors for Fish Products Safety and
Quality Controls ." J Bioprocess Biotech 13 (2023): 565.
Copyright: © 2023 Tarse DT, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Conventional glass electrodes and cutting-edge iridium oxide pH sensors were used to monitor fish product safety and quality controls. Because pH is used in so many chemical processes, practically all aqua samples have their pH checked at some point. So, amperometric or potentiometric devices are used in the most common pH sensor systems. Because of its excellent selectivity for hydrogen ions in a solution, durability, and ease of use, the most common potentiometric technique uses a glass electrode. Thus, tilapia fish samples were taken in the Gambella region's Baro River and promptly transferred to Addis Ababa, where they were held in the refrigerator until they were frozen. Mechanical unit operations were carried out at Wolkite university in food process engineering laboratories, Ethiopia. The liberated fish samples were dried in an oven before they were tested for pH at the JIJE Laboglass laboratory in Addis Ababa. Following the calibration of a standard solution with pH values of 4, 7, and 9.22, a solution made from a fish sample was calibrated, and the result was recorded on the pH meter. As a result, the pH values of the fish samples were 7.04 and 6.8 for direct and frozen samples, respectively. Hence, samples were reported as being at neutral levels. Due to the action of the quick-frozen method, or instantaneous freezing operation, the gapping problem after fish harvesting was negligible. Finally, it was determined that before conducting any nutritional parameters in the fish processing sector for the final finished containment, convenience, communicability, and protection of fish products, temperature, chemical activities, enzymatic activities, and microbiological deterioration must be managed. This is because fisheries require the most common processes used in the industry in the past, present, and future, so more scientific advancements should be made. In conclusion, prior to any processing unit operation, advanced pH measurement of fish safety and quality should be the primary application. As a result, it was the most effective approach for preventing fish and fish products from spoiling. In order to optimize the method, more research is needed to better understand the impact of proximal fish composition, post-harvest history, ambient conditions, microbial load, and the type and nature of bacteria. Therefore, pH testing is vital for fish safety and quality control of the finished products.
Fish product safety • Grading • Art of unit operation • Quality • pH sensor • IrO2 • Electrode • Food engineering • PH measurement • Sensor response mechanism
Rigor mortis is the process through which fish loses its flexibility due to stiffening of fish mussels after few hour of its death [1,2]. Measuring the pH of harvested fish is an important technique because it enables to know the level of the pH change that helps keeping pH stable and in optimal ranges reduces stress on workers even on product [3,4]. Hydrogen ion is ubiquitous species encountered in most chemical reactions [5]. It quantified in terms of pH the negative logarithm of its of its activity:
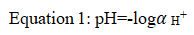
The pH sensors are widely used in monitoring safety and quality of foods. The earliest method of pH measurement was by means of chemical indicators, e.g. litmus paper that changes its color in accordance to a solution’s pH [6-9]. For example, when litmus is added to a basic solution it turns blue, while when added to an acidic solution the resultant color is red. Since many chemical processes are based on pH, almost all aqua samples have their pH tested at some point. The most common systems for pH sensing are based upon either amperometric or potentiometric devices [10]. The most popular potentiometric approach utilizes a glass electrode because of its high selectivity for hydrogen ions in a solution, reliability and straight forward operation. Ion selective membranes, ion-selective field effect transistors, two terminal microsensors, fibre optic and fluorescent sensor, metal oxide and conductometric pH sensing devices have also been developed [11]. However, these types of devices can often suffer from instability or drift and, therefore, require constant re-calibration. Although litmus indicators and other abovementioned pH sensors are still widely used in numerous areas, considerable research interest is now focused on the development of chemical or biological sensors using functional polymers. One may refer to a comprehensive review on the application of polymers in various sensor devices and more specifically, review of various methods used for pH measurement. By introduction of functional groups, polymers can be designed to selectively swell and shrink, thereby changing mass and elasticity, as a function of analyte concentration. The ion exchange properties of conducting polymers are of special interest for potentiometric-sensor development [12,13]. Conducting polymers are ideally suited for sensor applications because they not only exhibit high conductivity and electroactivity but they could also be used as a general matrix and can be further modified with other compounds in order to change selectivity. Compared to conductive polymers, nonconductive polymers usually have a high selective response and high impedance, which is important for eliminating interference by other electroactive species. Hence, measuring the fish pH can dramatically improve further processing operations such as cleaning, washing, descaling, deskining and deboning and ensuring the safety and quality of the final fish product. The pH of the flesh of freshly caught fish is usually close to 7.0, falling within a few hours of death, when rigor mortis has set in, it become to between 6.1 and 6.9 [14,15]. On the other hand, fish were sometimes gaping if they are frozen whole for thawing and filleting later even during operating different mechanical processes [16-20]. The pH change after harvesting fish greatly affects the mechanical unit operations. Following the death of fish, the flesh quickly becomes rigid, in a process known as rigor mortis. Rigor can affect the quality of whole fish in three main ways, by causing gaping in wet and frozen fish, and toughness and excessive drip loss on thawing in frozen fish. By measuring the pH of the fish and the levels of various compounds present the amount of autolysis that has occurred, and hence the freshness of the fish, can be measured. The major engineering interventions for fish post-harvest operations, processing and value addition are play a vital role of the final quality and safety of finished product. The fish intended during the measuring of pH value for further processing of mechanical unit operations should be of prime quality and in chilled condition. In addition to glycolysis, the pH change on fish (gaps) caused by handling, time after death, temperature, size and condition which furtherly affect fish mechanical unit operations. Moreover, the captured fish which may available via marketing are recommendable for their checking pH levels before further conducting various unit operations will be operated in food industry. This is because the gapped fish product will give poor finishing with extravagancy of the operation after labour, cost, resources, and time during manufacturing which affect the quality and safety of the fish products as a whole. Therefore, in this review experiment a practical measurement of the pH change before fish cleaning, de-scaling, deskinning and de-boning operation were conducted to ensure fish products quality and safety based ethical assurances of the food worldwide. On the other hand, advanced assessment on state of the art in iridium oxide based pH sensors for fish safety and quality controls was reviewed by the authors.
Since 1909, the pH measurement has been the most extensively used test to reflect the grade of solvents in chemical laboratories. As a result, pH sensors have become an important component in a variety of industries, including chemical, food, and biomaterials. The glass type electrode has evolved into a common tool for classifying the pH level of chemical solvents in the 90 years after it was originally used to assess hydrogen ion concentration. In a pH sensing test, the glass pH electrodes showed good sensitivity, selectivity, stability, and long life duration. Due to the physical nature of the glass membrane, however, glass electrodes have a number of drawbacks. Alkaline or Hydro- Fluoride (HF) acid is quickly affected or dissolved by the glass. Hence, to ensure quality and safety in fish products, visual inspection did not fill the confidence. In this review, some commercial technical applications which conventionally applied on fish after harvesting were studied before directly proceeded to pH measurements of a muscle because they are critical aspects as part of safety and quality controls.
Objective
• To ensure quality as the final objective of any quality strategy of fish processing operation that will be to reconcile needs/ specifications/actual production in all circumstances by immediate checking the pH value of dead fish.
• To assist the maintenance or improvement of profitability by minimizing customer complaints about quality, and hence to avoid the resulting lost business.
• To meet food safety and traceability requirements those reflect the desire of buyers and consumers to know where, how, and when the food on their plate will be produced in order to have a guarantee that it is safe.
Conventional and iridium oxide pH sensor apparatus, systems and methods used for pH measurements
The pH sensors are widely used in laboratories and industrial factories since many biological and chemical reaction mechanisms are pH dependent for the integral quality controls. ISO 9000 is an international standard that defines quality terms. The degree to which a set of inherent features meets requirements is characterized as quality. Quality is made up of a number of qualities, or components, that vary depending on the product or service in question. In the actual production process, a gap often appears between the real conditions and those foreseen in specifications (Venn diagram). At the intersection of the three circles, controlled quality can be found. Safety and quality assessment of fish processing operation can be managed via pH measurement which results save unit operations in food processing sectors (Figure 1).
Conventional glass type electrodes have been widely used in managing fish quality and safety; however, they still have certain disadvantages in specific applications. The glass rod sensor configuration is difficult to use for in vivo operation or food monitoring applications due to the brittleness of glass, size limitations and the lack of deformability.
Conventional pH sensor for measuring safety and quality of fish
The glass electrode is the most popular name for the measuring electrode. Thus, a thin delicate bulb of pH sensitive glass is used in traditional pH measurement electrodes. The glass electrode, which is generally sealed from the process and contains a seven buffer solutions, creates a voltage proportional to the hydrogen ion concentration in the process solution. The formula or law of Herman Walter Nernst can explain this relationship. For each decade of hydrogen ion concentration, the voltage changes by 59.16 mV at 25 degrees celsius, according to the calculations. At 7.0 pH, the isothermal point voltage is usually 0 mV. Heat and chemical attack have an impact on the performance of the glass electrode. The pH sensitive glass's inside and outer gel layers are both affected.
Inspection and pH status of fish processing operations
Visual examination: Fish were visually inspected for spoilage, flaws, and parasites, and defective fish were discovered and placed aside. Hence, the eye was much dimmed to the inside and more signs of gaps on its overall physiological appearances (Figure 2).
Measuring pH value of fish muscle before conducting de-boning in large scale
The pH meter was used to measure the pH value of the fish sample before and after freezing operation to determine the hydrogen ion concentration of a sample.
Procedure
• pH meters should be left plugged in and turned on all the time (Figure 3).
• Operation knob (button) should be on standby and electrodes stored in a 250 mL beaker with deionized water.
• PH meter should be “calibrated” before use and recalibrated after five or six readings. Fresh standard solutions should be used for calibration at the beginning of each lab and thrown out when the lab is over. The solution need not be changed during the lab period unless it becomes contaminated.
• To zero:
• Turn the temperature compensator to the temperature of the buffer being measured. Check to see if the test solution temperature is different.
• Carefully rinse electrodes with deionized water.
• Wipe off with Kimwipes.
• Resubmerge electrodes in a 50-mL beaker that is half full of a standard buffer with a pH of 7. Do not let the electrodes touch the bottom or sides of the beaker. Submerge electrodes carefully.
• Turn operation knob (or push button) from “standby” to “pH.” Digital display should read a pH of 7 but will probably need to be standardized. In order to standardize, turn “calibrate” knob until the display reads exactly 7.
• To make sure that the pH meter is working correctly, the electrodes must be resubmerged in another 50-ml beaker with a pH standard of either 4 or 10.
• Return pH meter to “standbs.” Lift the electrodes out of the pH 7 buffer, move the 50-ml beaker, and rinse electrodes with deionized water from a squeeze bottle. Dry with Kimwipes and resubmerge in the pH standard of 10 (or 4). Move operation knob from standby to pH. Recalibrate, if necessary, to the appropriate pH. If the display registers far away from 10 (or 4), see the instructor as the pH meter may be malfunctioning.
• To measure pH of test solution after zeroing:
• Turn the meter to standby.
• Remove electrodes, clean, and dry as before.
• Resubmerge in an adequate amount of test solution, turn pH meter to “pH,” wait 30 sec and record the reading.
• If the display “drifts” over a wide range, check with the instructor, as the pH meter may not be working correctly.
• To store electrodes between uses, thoroughly clean andw dry as before. Resubmerge in deionized water.
Managing safety and quality in fish processing unit operations
Prepare working area safe: Before beginning, the work space should be cleansed and sanitary standards should be maintained throughout. Examine, assess, and analyze work environments, as well as develop strategies and processes to control, eradicate, and prevent disease or damage caused by chemical, physical, and biological agents, as well as ergonomic variables. Inspections and enforcement of rules and regulations protecting individual health and safety are possible. Occupational Health and Safety (OHS) specialist positions in the commonwealth are divided into three career groups based on the nature of their work: Public safety compliance, environmental services, and minerals regulatory services.
Working principles of personnel to the quality and safety of fish cleaning, de-scaling, de-skining and de-boning operation
Assessment must confirm the ability to identify spoilage and common fish defects, diseases, and parasites for the species being cleaned, produce cleaned fish within enterprise productivity and food safety requirements that conform to state/territory food regulations and customer specifications, and knowledge of enterprise safe work practices, quality, and food safety requirements relevant to cleaning fish. Gloves, mitts, or gauntlets, as well as protective hand and arm covering; insulated protective clothing for freezers, chillers, and refrigeration units; non-slip and waterproof boots (gumboots) or other safety footwear; protective hair, beard, and boot covers; and uniforms, overalls, or protective clothing are examples of Personal Protective Equipment (PPE) (e.g. mesh and waterproof aprons.).
Principles of hygienic design of fish cleaning, de-scaling, deskinning and de-boning operation
By encouraging one standard design that would assist decrease contamination and associated recalls, optimizing the design and performance standards for equipment and related systems, as well as developing industry-wide guidelines, the entire industry benefits. These principles apply to all types of food equipment. According to the reviewed papers, there were ten sanitary design principles to control the quality and safety of products.
Hygienic design of fish cleaning, de-scaling, de-skinning and de-boning machines
Appropriate sanitary design was becoming more of a crucial concern in fish processing enterprises since it allows for the prevention of biological contamination of fish products as well as the enhancement of the desired final completed fish products' quality and safety. Furthermore, sanitary principles must be considered when designing and building product inspection equipment. As a result, fish cleaning, washing, beheading, evisceration, de-scaling, de-skinning, and de-boning machines were installed and operated using sanitary design principles similar to those used in the meat and poultry industries.
Working principle of fish cleaning, de-scaling, de-skinning and de-boning machines
Manual, semi-automated (commercial), or automated setup processes were utilized as per processing scale and availability of materials in mechanical unit operations. Band saws, de-boning machines, fat suction tools and equipment, fish cleaning troughs, fish tubs and bins, gutting machines, hand-held scalers, knives, scaling knife, scaling machine, and weighing scales are examples of possible equipment (Figure 4).
(1) Line lamp; (2) wrong cyclic sense; (3) start pushbutton: The green start button is pressed to start work. This button activates the motor and, therefore, the cutting unit conveyor belts. (4) Stop pushbutton: Stop button stops the motor and, therefore, the cutting unit conveyor belts. (5) Main switch: This switch commands the high voltage to the motor and the control circuit transformer. It is found on the electrical panel. When this switch is turned to 0 (zero), the electrical current to the machine is interrupted completely. It is the only switch which interrupts the electricity completely and totally, and this switch must be turned to zero (0) before starting any cleaning or maintenance operations (Figure 5).
The models have been designed and constructed for squid, cuttlefish, polyps which are to be cut in rings, strips or sections. The following assessment method is suggested: Observation of practical demonstration and oral questions.
Mechanical unit operation in fish processing
Fish sorting and grading: Normally, fish between the sizes of 20 and 50 cm can be simply de-boned with a de-boning machine. The gut would flow through the machine with the flesh if the whole ungutted fish was pushed through the meat bone separator. This would result in a non-white mince including skin, liver, kidney, intestinal contents, blood, and pigments, among other things. Apart from color issues, microbial infection can cause toxicological issues in mince. Moreover, the fish mince would be of poor quality. As a result, the viscera and head must be removed before the fish will be placed in the de-boning machine.
Fish cleaning: Cleaning and preparing fish to customer specifications was done securely with cleaning equipment. The scales were removed, leaving the skin unharmed, and the fish was rinsed in potable water to remove any loose scales. The gills and guts were removed without cutting into the flesh, and the fish was rinsed in potable water to eliminate any loose debris. The appropriate container was used to place the fish components for further processing or disposal. Cleaning fish satisfies business productivity criteria. According to the working instructions, cleaned fish were displayed or stored. Through accurate and compliance labeling, product identification and traceability are maintained for quality and safety assurance.
Fish de-scaling: Fish scales were automatically removed using a fish scaling machine. Fish scale machines were appropriate for a variety of fish, including tilapia, carps, grass carps, groupers, crucians, and chubs, among others.
De-scaler for fish with variable drum speed: Fish de-scaling equipment was designed and manufactured to easily remove fish scales. Scales can be removed from practically all types/sizes/ species of fish, including marine and freshwater species such as Sardine, Tilapia, and Rohu. The machine was built of stainless steel 304 and has a capacity of 10 kg. It has a 1.5 HP induction motor and a Variable Frequency Drive (VFD) that allows the drum speed to be adjusted based on the type of fish loaded. Perforated SS 304 sheet is put in the drum (Figure 6).
Table-top fish de-scaling equipment with fixed drum speed: Fish de-scaling equipment was designed and manufactured to easily remove fish scales. Scales can be removed from practically all types/ sizes/species of fish, including marine and freshwater species such as Sardine, Tilapia, and Rohu. This machine was also built of stainless steel 304 and has a capacity of 5 kg. It has a 0.5 horsepower AC motor with a belt reduction system to produce the desired drum speed of 20-30 revolutions per minute. The body was made of one-inch square SS dismantling tube with an appropriate covering in the electrical sections. The drum was composed of perforated stainless steel sheet and was housed in a sturdy stainless steel frame with scale-removal projections and a leak proof door.
Fish de-scaling machine hand operated: Fish de-scaling equipment was designed and manufactured to easily remove fish scales. This apparatus can remove scales from a wide variety of fish species, including marine and freshwater species such as sardines, tilapia, and rohu. This machine was built of stainless steel 304 and has a capacity of 5 kg. The body was made of dismantling type 1 inch square stainless steel tubing. The drum, which has a diameter of 255.5 mm and a length of 270 mm, is made of perforated SS sheet and is mounted in a sturdy SS frame with scale-removal projections and a leak proof door with an appropriate lock. To manually rotate the drum, a pedal is installed on the side.
Fish de-skinning: Skinning operation was conducted manually, batch or automatic operation which depended on the following factors which affect fish quality and safety of the final product.
Handling: An hour or two after catching, the fish become stiff in rigor because the muscles pull against each other. This puts the connective tissue under some strain and, when a fish that has stiffened in a bent position is wrenched straight before it is put into the freezer, the connective tissue tears and causes gaping. Similarly, any rough handling of the fish, for example by throwing them against the washer or standing on them as they lie in the pound, can cause damage that may result in gaping.
Time after death: Gapping will takes place as whole fish stayed at ambient condition still yet arrive at market after catching time. When whole fish are frozen before the onset of rigor, fillets cut from them after thawing are least likely to gape. When whole fish are frozen during rigor, even though they are handled gently, the fillets will gape to some extent, and when the fish are frozen after they have passed through rigor, fillets taken from them will gape still more; the staler the fish are before freezing, the more the fillets will gape.
Temperature: The connective tissue of newly caught fish is very sensitive to small rises in temperature; it is weakened after only 4 hours at 17ºC, the temperature of a mild day. At 20ºC it weakens more quickly, and fish kept at 25ºC before freezing will almost always gape severely. When a newly killed fish is warmed by lying on a warm deck for half an hour, it quickly stiffens in rigor, and the strong muscle contraction breaks the weakened connective tissue, so that at 20°C or more severe gaping results from the fish going into rigor even before it is frozen; freezing and thawing make matters worse still. When the fish are warm, any handling such as gutting, washing or moving them about almost invariably results in severe gaping. However, when warm fish are cooled again in ice or cold sea water, the connective tissue recovers most of its strength. Provided the warm fish did not go into rigor, rapid chilling will enable the fish to be handled in the usual way without increasing the risk of gaping. If the temperature reaches 30° C, as it quickly can on a warm deck, then subsequent cooling will not help much; the connective tissue will have been damaged too badly.
Size: For fish in the same biological condition, and given the same treatment on board, small ones gape more than big ones; the explanation is that probably the thicker connective tissue of large fish gives them a structural advantage.
Condition: Fish in good condition are smooth and firm to the touch, and kick furiously about the deck after capture, whereas fish in poor condition are lean, perhaps loose skinned, and lifeless; the cut surface of a fillet from a fish in bad condition feels watery. Fish lose condition at the spawning season. Since most white fish species spawn in the spring and thus make their eggs or sperm in winter when food is scarce, they rob their own body resources and so cause the fillets to become soft and watery. This softness does not cause gaping; watery fish often have a completely smooth fillet surface, because the connective tissue becomes stronger at the spawning period. However, there is a change in the chemistry of the muscle after spawning, when the fish begin to feed heavily again at the beginning of the summer, and the fish is then very liable to gape. This is the time, more than any other, when the fish must be kept cool and handled gently in order to keep gaping at a minimum.
Shape: Round fish gape more than flatfish and each species have its own characteristic degree of gaping.
Fish de-boning: A fish meat bone separator with Variable Frequency Drive (VFD) was created and developed to extract pin bones from freshwater fishes. This may be utilized at speeds ranging from 5 to 100 rpm. With the development of a novel belt tightening method, the new machine may be simply converted to any species and does not require specimen customization during the design stage. Only two speeds are available in existing imported versions, limiting yield efficiency in a single span operation and making it difficult to alter the system to use specimens other than those for which the yield was initially customized.
Iridium oxide based pH sensors for fish safety and quality controls
To achieve small sizes and robust design, Ion-Sensitive Field-Effect Transistor (iSFET) pH sensors, optical fiber pH sensors, hydrogel film pH sensors, and solid sate pH sensors have been proposed. pH sensors are classified according to their capabilities and limits.
The pH sensors are classified as Ion-Sensitive Field- Effect Transistor (iSFET) pH sensors, optical fiber pH sensors, hydrogel film pH sensors, and solid sate pH sensors. Ion-Sensitive Field-Effect Transistor (iSFET) sensors have power consumption concerns due to the Field-Effect Transistor (FET) operational requirements. Hydrogel film pH sensors utilize the physical properties of the pH response swelling and shrinking polymer to measure resistance changes. The sensor structure design and polymer layer fabrication process can be complicated and expensive. Optical pH sensors also have power consumption issues due to the use of light sources. The system including optical devices could be expensive and unsuitable for implantation. Various solid-state metal oxides have been investigated for pH sensing electrodes including PtO2, IrOx, RuO2, OsO2, Ta2O5, RhO2, TiO2 and SnO2 as the pH sensing films. The pH sensitivity, selectivity, working range, and hysteresis indicate sensing performance. IrOx, RuO2 and SnO2 have been demonstrated with more advantages in sensor performance for various applications. RuO2 and SnO2 show near Nernstian responses in wide pH ranges. However, SnO2 and RuO2 presented hysteresis and drift problems leading to potential calibration issues and unstable responses. Therefore, Iridium Oxide Film (IROF) has performed outstanding stability over wide pH rages, rapid responses, less hysteresis and high durability, which have also been demonstrated at high temperature up to 250ºC.
Background art of iridium oxide based pH sensors for fish safety and quality controls
Because of their remarkable stability over a wide pH range, rapid reactions, low hysteresis, and good durability, iridium oxide based pH sensors were more effective in fish quality controls than the others. As a result of the many fabrication possibilities and procedures for Iridium Oxide Films (IROF), iridium oxide based pH sensors are preferred over other sensors for regulating the safety and quality of fish products beyond traditional monitoring electrodes based pH readings. For the manufacture of Iridium oxide based pH sensors, there are a variety of cost-effective and High-Quality Iridium Oxide Film (IROF) fabrication methods. During the experiments conducted by other researchers, the oxygen and argon pressure ratios, target position, deposition rate, and RF powers were all measured.
Apparatus and technical methods: Despite the fact that Srensen's idea of pH originates from the turn of the twentieth century, using a Harned cell to determine absolute pH values in aqueous and non-aqueous liquids remains highly circumstantial. As a result, all proton probes now in use, such as the glass electrode and Ion Selective Field Effect Transistors (ISFETs), are calibrated using standard solutions.
Attempts to replace the potentiometric approach with coulometric proton counting remain speculative. The following items are included in the scope of this review article:
• The analytical meaning of pH.
• Classic and novel pH measurement techniques.
• The wide range of pH sensitive materials.
• The various preparation procedures documented in the literature.
Singlerod measuring cell with double junction: 1=solution, 2=inner electrolyte (KCl, 3 mol/L, pH 7), 3=reference electrode, 4=external Ag|AgCl|KCl reference electrode, 5=junction.
Ideal chain voltage versus pH.
The double junction electrode introduced an additional chamber between reference electrode and external solution to save the reference electrolyte from external contamination (Figure 7). Only two reference electrodes can determine the potential difference I–II across the thin glass membrane, which reflects the difference in H+ activities on a both sides. The soaking layers on both sides of the membrane, which are less than 0.5 m thick, allow cations in the silicate framework to exchange with H3O+ from the surrounding solutions and vice versa. The thin glass membrane's cationic conductivity connects the two soaking layers. The membrane contribution and the diffusion potentials at the liquid junction between each reference make up the measured chain voltage E. The modest nonrandom variation in output voltage with time (by a few mV h-1) in a solution with constant composition and temperature is known as the drift effect. The measurement circuit, reference electrode, and device body are all impacted significantly. Different output voltages at the buffer solution insulator interface occur when the ISFET is tested numerous times in the same pH buffer solution. This is known as hysteresis or memory effect. In different pH buffer solutions, this apparent delay of the pH reaction causes a loop cycle with variable hysteresis widths. When the light is switched on, the output voltage of the ISFET changes (by about ten mV) (Table 1).
|
Range of application |
Challenges |
|---|---|---|
Glass electrode |
Temperature: <80. 130ºC Pressure: <60 bar (with counterpressure) Stability: +1 mV week" |
1) Interaction of surfactants and film formation on theglass surface in reaction mixtures. |
ISFET |
Temperature: <85 CPressure: <2 bar |
1) Film formation on the surface. |
Antimony electrode |
e.g. strong caustic solutions (no sodium error), fluoride containing waste water. |
1) High degree of asymmetry (pHiso=-3). |
Optical sensors |
Transparent liquids Small and flexible (fiber sensors) no reference element required signal transmittance over large distances. |
1) Change of transparency of the solution. |
Electrodes with gel membrane: Proton-sensing compounds in a polymer matrix, such as N-octadecylmorpholine, were ideal for pH measurements in a restricted pH range.
Monolayers with electrochemical activity: The pH variations may be indicated by redox-active groups in a receptor adsorbate on a conducting substrate. The alkyl groups may be connected to a ferrocene group and a carboxyl acid group, e.g. (C5H5), Fe (C5H4)– (CH2)6–S–(CH2)7COOH. Thioethers adsorb strongly on a gold surface through the sulfur atom. The redox peak of ferrocene in the cyclic voltamogram shifts by roughly 200 mV when the pH is changed from 6.5 to 1. Organic guest molecules can be bound by cyclodextrins, calixarenes, and cavitands in a hydrophobic cavity, which can be used in biosensors. Storage electrodes and hydrogen electrodes are two types of electrodes that can be used to store hydrogen. Platinum black is most corrosion resistant when purged with pure hydrogen, although it is easily poisoned by CN–, H2S, and As2O3. If platinum is not kept under hydrogen for an extended period of time, it absorbs oxygen and reduces heavy metal ions, nitrate, and nitrophenols on its surface. Palladium, ruthenium, and osmium, when resaturated with hydrogen on a regular basis, can be used as pH probes without the need to keep them in a constant current of hydrogen (such as platinum). It has a pH of E=–0.0591 (at 25ºC), or pH=–16.92 E. The half-standard cell's potential indicates whether a redox system is stable in aqueous solution or if it functions as an oxygen electrode (E0'> 0.815 V at pH 7) or a hydrogen electrode (E0'–0,414 V at pH 7) in aqueous solution. Metals having a standard potential E0<0 V dissolve in aqueous solution. Values in parentheses denote unstable oxides at these conditions. In alkaline solutions, hydroxides are existent (Table 2).
| Group | Material | Redox equilibrium: Ox+ze- ⇌ Red | E0/V (pH 0) | E0’/V (pH 14) |
|---|---|---|---|---|
| IVa | Tin | SnO2+4H++4e- ⇌ Sn+2H2O | -0.117 | -0.945 |
| Lead | HPbO2-+H2O+2e- ⇌ Pb+3OH- | (-0.36) | -0.537 | |
| Va | Arsenic | As2O3+6H++6e- ⇌ 2As+3H2O | +0.234 | -0.68 |
| Antimony | Sb2O3+6H++6e- ⇌2Sb+3H2O | +0.152 | -0.639 | |
| Bismuth | Bi2O3+3H2O+6e- ⇌ 2Bi+6OH- | +0.317 | -0.46*) | |
| Ib | Copper | Cu2O+H2O+2e- ⇌ 2Cu+2OH- | (+0.34 ) | -0.36*) |
| Silver | Ag2O+H2O+2e- ⇌ 2Ag+2OH- | (+0.80 ) | +0.342 | |
| Gold | H2AuO3-+H2Q+3e- ⇌ Au+4OH- | +1.50 | +0.70 | |
| IIb | Zinc | ZnO+H2O+2e- ⇌ Zn+2OH- | (-0.497) | (-1.26)* |
| Mercury | HgO+H2O+2e- ⇌ Hg+2OH= | +0.860 | +0.098 | |
| Vb | Tantalum | Ta2O5+10H++10e- ⇌ 2Ta+5H2O | -0.750 | -1.578 |
| VIb | Tungsten | WO2+4H t 4e W+2H2O= | -0.119 | (-0.946) |
| VIIb | Rhenium | Re2O3+6H++6e- ⇌ 2Re+3H2O | +0.227 | -0.600 |
| VIIIb | Iron | Fe3O4+8H++8e- ⇌ 3Fe+4H2O | (-0.085) | -0.912*) |
| Nickel | NiO+2H++2e- ⇌ Ni H2O | (+0.110) | -0.717*) | |
| Osmium | OsO4+8H++8e- ⇌ Os+4H2O | +0.838 | (0.00) | |
| Rhodium | RhOH2++H+3e- ⇌ Rh+HO | +0.83 | 0.00 | |
| Iridium | Ir2O3+3H2O+6e- ⇌ 2Ir+60H | +0.923 | +0.098 | |
| Platinum | PtO2+4H++4e- ⇌ Pt+2H2O | +1.0 | +0.14 |
The antimony electrode's measured cell voltage versus a reference electrode is disrupted by reducing and oxidizing chemicals in the solution, which is an issue that all metal electrodes face. The bismuth electrode has been described in alkaline solutions. With Sn, W, Fe, Ir, Os, Ag, Cu, Zn, and other metals, almost linear functions of potential vs pH were reported, primarily in oxygen free buffer solutions. The slope rarely equals the theoretical value of (ln 10) RT/F, is limited to a narrow pH range, and is dependent on the anions present in the solution. These electrodes aren't just hydrogen electrodes; they're the result of numerous simultaneous potential-determining processes. Faradaic delivered charges Q from the battery like redox processes involved in the potential determining charge transfer reaction across the interface always superimpose the Helmholtz double layer capacitance. In the disrupted rutile lattice, water molecules saturate the free valences. The RuO2 surface is covered by hydroxide groups due to dissociative adsorption of water, as illustrated in Figure, which strive to form oxide sites by releasing protons. The purpose of the method could be to correct for the oxide's oxygen defect stoichiometry.
Electrodes of reference: The Standard Hydrogen Electrode (SHE) has a set of experimental criteria that aren't easy to meet. Due to environmental concerns and mercury toxicity, the most stable calomel electrode (Hg|Hg2Cl2|Cl–) is no longer used. For chemical sensors, the Ag|AgCl|KCl (3.5 mol/L) reference electrode is typically utilized. However, liquid filling makes miniaturization and applications at greater pressures and temperatures more difficult. Electrode made of silver chloride: The Ag|AgCl|Cl– electrode functions without AgCl on its surface; AgCl can be disseminated in the solution, but not on the electrode.
Measuring techniques: Traditionally, the potentiometric method is favored for pH measurements. Additionally, amperometry has been established especially for biosensors. The coulometric determination of proton concentrations offers for future sensor applications, e.g. based on redox active metal oxides. However, much work has to be done to finally correlate the faradaic redox reactions, which directly depend on pH, from all capacitive surface effects which reflect the electrolyte electrode interface and the composition of the surrounding solution. As a preliminary step to a future direct recording pH sensor, ac impedance spectroscopy might be useful. This technique allows the separation of electrolyte resistance, charge transfer processes and diffusion processes along the grain-boundaries and in the threedimensional pores of the material. Tungsten trioxide, in a mixture with iridium oxide is used in a capacitive pH sensor. Thus, the solution resistance Rel is subtracted from the measured impedance to exclude both the geometric dimensions of the sensor and the ionic conductivity of the solution. Then the frequency-dependent interfacial capacitance Cp (ω), corrected by the solution resistance, is calculated for each frequency f from the measured real and imaginary parts according to equation 2 as follows:
Equation 2: Cp ((ω)=-ImZ/2π((ReZ-Re1)2-(ImZ)2)
If the dc resistance R of the pH measuring cell is assumed to be large, it holds CS (ω)=(2πf Im Z)–1 ≈ CP at low frequencies. Then the parallel equivalent circuit Rel–CP||RP simplifies to a series combination, of Rel–CS. Both Cp and Cs are frequency dependent differential capacitances. The pH sensor may work either at a given frequency or differential capacitance is averaged by integration in a given frequency range. The change of resistance and capacitance may also be used in commercial pH meters as a diagnostic tool for the aging of a pH electrode and the requirement for re-calibration.
Iridium oxide based pH sensors for fish safety and quality controls
Iridium (Ir) has been established as a noble metal that is stable in aqueous solutions at all pH values. Iridium is extremely resistant to harsh chemical reagents such as caustic alkali aqueous solutions, acids, and oxidizing agents. Iridium is most likely formed in a finely split state by the creation of complex ions with a valence of +3 (ex. Ir2O3) or +4 (ex. Ir2O4) (ex. IrO2). At 25 degrees Celsius and atmospheric pressure, iridium can absorb 807 times its own volume of hydrogen. Iridium is rarely damaged when employed as an anode, even in the presence of chlorine produced by electrolysis. When an iridium anode is polarized, oxygen is deposited onto the metal surface (Figure 8).
Thermal and electrochemical preparation procedures are the standard process in many fabrication processes to generate IrOx. In general, electrochemical fabrication can be used to create Anodic Iridium Oxide Films (AIROF). The basic properties of these types of iridium oxide films are their poorly crystallized structure and highly hydrated surface. The thermal preparation method is used for sputtering iridium oxide (SIROF) and Sol-Gel (SG) IrOx. The fabrication process necessitates annealing oxidation. The shape of iridium oxide will alter depending on the heating profile and temperature point. The electrolyte influences the shape of the hydrous IrOx film. This type of IrOx is hydrous, porous, and has a high degree of water, proton, and other ion transport. However, the current must be precisely controlled in this fabrication process, and the potential must surpass key upper (Eg+) and lower (Eg-) potential limitations during each deposition cycle. This fabrication necessitates the use of an expensive potentiostat and a pure iridium oxide substrate. The use of sputtering to deposit IrOx electrodes has grown in popularity. The SIROF deposition technique, on the other hand, is expensive due to the target cost. The SIROF are amorphous and have a characteristic rutile appearance at room temperature.
Process of fabrication of sensors
Standard photolithography and lift-off procedures were used to create our pH sensor. Electron beam evaporation was used to deposit all of the metal layers. On a piece of Kapton polyimide substrate, a layer of 7 nm thick Cr was deposited first, followed by a 0.1 m thick coating of Au. The sol-gel procedure was used to create the iridium oxide sensing film. For adhesion, 7 nm thick Cr and 3 nm thick Pt were evaporated. Electron beam evaporation was used to deposit a 30 nm thick silver coating. Electroplating was used to create Silver Chloride (AgCl) reference electrodes (Figure 9).
Sol-gel Iridium oxide process
The sol-gel coating solution was created using the method specified in for rigid substrates. One gram of anhydrous Iridium Chloride (IrCl4) was dissolved in 42 milliliters of Ethanol (C2H5OH), and then 10 milliliters of Acetic Acid (CH3COOH) was added to the solution. A magnetic rod at the bottom of the beaker was used to swirl the solution constantly for at least one hour. The flowchart of the IrOx sol-gel method is shown in Figure 10. The chemical reaction went like this:
Formation of a film: There are several ways to cover the thin layer on the substrate in the sol-gel process, including spin coating, dip coating, and spraying. The spin coating process is commonly used with condensing agents such as polymer or photoresist. The advantage of spin coating is that during the spin-off process, a film of liquid tends to become homogeneous in thickness. Once the surface is uniform, the thickness tends to stay the same, and the thickness does not diffuse differently depending on the substrate. Deposition, spin-up, spin-off, and evaporation are the steps in the spin coating process. Our coating agent, on the other hand, is based on ethanol, which is easily dissipated in our application.
Description of the IROF used in Iridium oxide based pH sensors for fish safety and quality controls: The terms "a,""an," and "the" do not relate to a single thing, but rather to a broad class of which a specific example can be used to illustrate. The terminology used herein is intended to describe specific embodiments of the application, but it is not intended to limit the application beyond what is stated in the claims. The sol-gel method was used to create an iridium oxide sensing film. Cr with a thickness of 7 nanometers and Pt with a thickness of 3 nanometers were used.
Hanna pH meter: Authors of this review are highly recommending the readers to revisit the state of the art of Hana pH application site that shows how to use and calibrate the Hanna Instruments HI 98107 pHep pH Meter. Versatile pH meter for agriculture, fish farming, water quality, food manufacturing and more (Figure 11).
Experiment location
Tilapia fish samples were collected in Baro River in Gambella region and immediately transported to Addis Ababa and stored under refrigerator for freezing. The frozen fish samples were dried under oven for further pH measurement at JIJE Laboglass Laboratory. Furthermore, the mechanical unit operations were carried out at Wolkite University in Food Process Engineering Laboratories, Ethiopia (Table 3).
| Whole fish (Tilapia) | Mini blender | Flasks |
|---|---|---|
| Ice box | Desicator | Beakers |
| Potable water | Plastic trays | Dishes |
| Processing table | Scissors | pH buffer solutions (4,7,9.2) |
| Analytical balance | Air blast freezer | Distilled water |
| Cutting boards | Deep Freezer | Manual de-scaling material such as knife, |
| Refrigerator | pH meter (CP-505) | Grinding pestle and mortar |
| Aluminum foil | Gloves | |
| Face mask | Gown | |
| Potassium chloride (Kcl) | ||
| Electric thermostatic heated dry oven with Equip No: 041 (202-1 AB). | ||
| Mechanical unit operation facilities for de-scaling, de-skinning and de-boning. | ||
Materials and chemicals
Working principle
In order to ensure fish products safety and quality control; checking pH value of the fish products should be the preliminary test for the further mass and automated mechanical unit operations such as descaling, de-skinning and de-boning. This is because in mass processing it is impossible to proceed to automatic process after the immediate boarding of the fish from harvesting source. Hence, fish fmay stay a while in ambient condition or Individually Quick-Frozen (IQF) method may apply before processing. These possibilities cause problems of fish product gape which lead to poor quality and safety of the final products as mechanical unit operations will be applied for the further processing. Therefore, conducting de-scaling, de-skinning and deboning operations after the result of pH measurement enables the processor to avoid gapes (problems occurring in fish thus affect mechanical unit operations and overall quality) for the efficient, safe and quality manufacturing of fish products.
Procedures
The pH testing activities were primary conducted by the analyzer before the mechanical unit operations (automatic de-boning). Thus, these pH testing activities were demonstrated mainly for capture fishery because the sources (availability) of fish might be from markets to ensuring safety and quality before the fish products preceded to automatic de-boning. Hence, fish handling was conducted with the situation of distance from markets, time after death, temperature, other variable conditions, size and shape of the fish. This experiment was carried out to determine the range of time from death to the onset and to resolution of rigor for fish. The processing procedure for measuring rigor index is shown in Figure 12.
The cuttingers method (tail drop), developed was used. The rigor index (Ir) was calculated using the following formula:
Equation 3: 4Ir=(L0-Lt/L0) × 100
Where; L represents the vertical drop (cm) of the tail, when half of the fish fork length is placed on the edge of a table. L0 is the tail drop at the beginning of the experiment, while Lt represents measurements throughout the experiment. There were two possibilities in filleting the fish. In the first step cutting the muscle from one side of fish and taken off from bone and recorded as F1. On the other hand turn the other side of fish did the similar step and recorded as F2. F1 fillet was directly measured for their pH value while pH value was measured after the F2 fillet was further frozen and thawed for their pH change. Furthermore, the fish were measured immediately after capture and cranial spikes were placed on a horizontal table in such a way that half of its body (the tail part) remained outside of the table. At selected time intervals, the rigor index was calculated by the following formula:
Rigor indexed (%)=Do-D/Do×100
Where, Do and D represent the distances of the base of caudal fin from horizontal line of the table at the start of the experiment and at subsequent storage periods, respectively.
Procedures for the pH testing
Sample drying
• Set the temperature of air oven to 130ºC.
• Dry an aluminum weighing dish ≥ 1 hr at 130ºC.
• Cool and store dry dish in a desicator.
• Cool for ≥ 30 minutes before using. Covered weighing dishes are useful when analyzing samples that splatter. weighing dishes without covers may otherwise be preferred, as they are disposable.
• Weigh empty dried dishes (with cover, if used) to nearest 0.1 mg.
• Add 3-10 gm homogeneous sample to dish and weigh (with cover, if used) to nearest 0.1 gm.
• Place dish with sample into oven and dry for 2 hours.
• Remove dish with dried sample and cool 30 minutes in a desicator.
• Weigh cooled dishes with dried sample.
• Return dish with sample to oven, dry for another hour, cool and reweigh.
• If weight has not changed, test is done. If weight is lower, continue drying for 1 hour periods and reweighing until constant weight is achieved.
• Depending on the sample, drying times can be ≥ 16 hours.
Calculations
Calculate moisture as the percent loss in weight after drying
% Moisture content=(Wt of wet sample in gm)-(Wt of dry sample in
gm)/Wt of wet sample in gm ×100
Sometimes a related parameter, known as the total solids, is reported as a measure of the moisture content.
Sample grinding
1st grinding was conducted by mini grinder. Thus the sample which dried at 600C for 2 hours was ground for its Dry Matter (DM) determination.
% dry matter=M Dried/M Initial × 100 or % Dry Matter=(100-% Moisture)
Remark: to obtain an accurate measurement of the moisture content or total solids of a food using evaporation method, it is necessary to remove all of the water molecules that were originally present in the food, without changing the mass of the food matrix.
2nd grinding was conducted by taking the re-dried (at 120ºC for 3 hours and left for 1 hour in air circulated incubator) samples using pestle as and mortar.
Making a solution of sample
• 1:1 ratio (w:v); 5 gm of the ground sample was measured by sensitive balance and add into the 5 mL distilled water to make a solution.
• Mix using glass rode by shaking it for 30 minutes and cover the solution and left as stock solution for 1 hour to make the solution stable.
• Filtrate the solution using filter fennel to separate impurities. Use the filtered solution for the pH calibration.
Measuring the pH of the sample
Analytical balance
• Glass beaker: 100 mL.
• Shaking bottle.
• Mechanical shaker.
• pH meter with combination of electrode with Kcl internal reference and auto-temperature compensator.
• Wash bottle.
Reagents
• Standard buffer or pH=400; 700 and 900 ppm.
• Sistilled water.
Procedure
• Weigh 10 gm of homogeneous and ground at <4 mm particle size not more than three minutes in 2000 rpm flour sample from a freshly opened package into a 250 mL shaking bottle.
• Add 100 mL of freshly distilled water and closed the stopper. This is 1:10 ratio.
• Shake until particles are evenly suspended mixture is free of lumps.
• Place shaking bottle on a mechanical shaker and shake for 30 minutes in a swirling motion.
• Remove the shaking bottle from the shaker and allow standing to an additional 10 minutes.
• Decant the supernatant into a 100 mL glass beaker and ready for determination of pH
• Before any calibration or measurements are to occur.
• Then, calibrate the pH meter using standard buffer solution of 400,700 and 900 at three point calibration with temperature compensation.
• Insert the electrode and auto-temp compensation probe into the beaker containing the supernatant and ensure that the level of solution is above the junction point of the pH electrode.
• Read pH.
Procedures for mechanical unit operations (de-scaling, deskining and de-boning)
Check, sanitize and calibrate equipment for the fish processing methods in accordance with manufacturer’s specifications thus prepare and sanitize utensils.
Fish de-scaling procedures
• To descale the fish, put it inside a large plastic bag, head first (to prevent the scales flying everywhere). Using a fish filleting knife upside down (the non-sharp side against the fish) and holding onto the fish tail, push backwards towards the head and the scales should flip off. Turn the fish over and repeat on the second side.
• Once the scales are removed, take the fish out of the plastic bag and rinse under cold running water to remove any loose scales. Pat dry with kitchen paper.
• Using a pair of kitchen scissors cut off all the fins except the dorsal fin (in the middle on the back). The tail can be trimmed into a ‘V’ shape if it needed.
• Remove the gills on both sides, by opening the gill flaps and cutting round the back of the gills towards the body of the fish from the top of the fish down towards the belly.
• To gut the fish, place one hand on the uppermost side of the fish to help keep the belly skin taut and insert the point of a filleting knife into the vent hole.
• Cut up the middle of the belly of the fish to just behind the head. Don’t push too deep with the knife or the guts will be cut into unnecessarily.
• Open the belly and pull away the guts, including the heart and liver tucked just behind the head. You might need to snip these out using a pair of scissors.
• Once the guts are removed, break the blood line with the handle of a teaspoon and scrape out all the blood under the back bone.
• Rinse the fish and belly area well under cold running water to remove any blood. Wipe the fish out with kitchen paper. It is now ready to cook whole or to fillet.
Fish de-skinning and deboning procedure
• Wash the whole fish in potable water and keep it on a cutting board.
• Cut behind the head while angling the knife towards the front of the fish. Cut down to the bone till the knife reaches just behind the pectoral fins.
• Turn the fish and run the knife just clear of the dorsal fins with a slight downward angle until it reaches up to the backbone.
• Peel the fillet back and run the knife over the backbone for severing the small lateral bones.
• Turn the fish over and repeat the first cut made behind the fish head on the other side.
• Repeat the second cut near the dorsal fin with the knife angled slightly down. Continue this along the length of the fish.
• Reverse the direction of the filleting knife and follow the bones by “feeling them” with the fillet knife until the backbone is reached.
• Peel the fillet back and cut around the backbone and through the small lateral bones. Run the fillet knife right through to the skin on the underside of the fish.
• Cut over the belly flap either through or over the belly bones. Cut any remaining attached muscle or skin.
• Remove the first fillet. Flip the fish back to the other side and cut the bones around the gut cavity. Release the second fillet from the backbone. Now there are two fillets separated from the body of the fish.
• To de-skin the fillet, first lay the fillet skin side down and carefully cut between the fillet and fish skin at the tail end while holding the knife at a shallow angle. Stop when inch or two of fillet portion is released from the skin.
• Hold the released portion of the skin firmly with forefinger. Change grip on the fillet to a secure grip on the tab of fish skin created with the first cut.
• Firmly hold the knife still and at a fixed angle between the fillet and the skin and wriggle the skin from side to side while pulling backwards on the tab of fish skin.
• Continue this motion through the fillet. The fillet and skin are parted to get a de-skinned fillet.
• The fillet will have a belly flap attached to it. The belly flap also has to be cut and removed to reduce the bone content.
• Trim the fillets to uniform size. The frames, belly flaps, trimming wastes can be cooked to extract meat which can be utilized for the preparation cooked mince based fish products viz. cutlets, fish wafers etc.
• The de-skinned and deboned fish fillets can be packed in thermofoam containers or laminated pouches which can be processed as IQF products or can be block frozen and stored as raw material for the preparation of coated.
Results from conventional PH calibration and freezing operation
Following the standard solution of the pH value 4, 7 and 9.22 calibrations, a solution prepared from fish sample was calibrated and recorded the result from the pH meter. Consequently, pH values of the fish samples were recorded as 7.04 and 6.8 of direct and after freezed respectively. Hence, samples were reported as at neutral levels. Due to the action of the quick-frozen method or instantaneous freeing operation, the gapping problem after fish harvesting was negligible. However, as the freezing operation was not completed quickly, the fish suffered more severe gaping as a result of the fish becoming rigid before being frozen; freezing and thawing exacerbated the problem. Although, the pH value was safe after quick freezing actions, strong muscle contraction breaked the weakened connective tissue and hinder the application of de-scaling, de-skinning and deboning for the further large scale industrial operations. Some of the fish were not quickly frozen which then caused gapping as whole fish stayed at ambient condition which reduced the quality and safety of the product. Since the size and shape parameters of the fish were elected based on appropriateness of the further equipment operations, there were no adverse effects due to size and shape in this experiment. In general, the pH values of 7.04 and 6.8 indicated that, it was a positive possibility outcome resulted from the experiment in order to conduct de-scaling, de-skinning and de-boning unit operations in fish processing industry in large from the conational pH measurement similar results were reported by different authors. It created the confidences in minds of the processors regarding the ethical approvals and concerned responsibilities for the expected safe and quality finished product. Therefore, the pH values of 7.04 and 6.8 illustrated the positive expectation on fish products quality and a safety assurance as it was forecasted for the final finished products.
Results of some mechanical unit operations (de-scaling, de-skinning and de-boning)
Principles of Occupational Health and Safety (OHS) applications were critical measures after the achievement of the pH measurement for the fish processing safety and quality controls in food industry. The OHS governed the principles of de-scaling, de-skinning and deboning operations in fish processing sectors. Since, the physical, chemical and biological hazards and risks associated with these mechanical unit operations were managed properly; it was a bright ambitious safety and quality products. The changes in pH value of fish during the rigor mortis have been monitored by occupational health and safety principles in parallel with principles of hygienic designs. De-scaling, de-skinning and de-boning machines in Food Process Engineering Laboratories at Wolkite University were operated manually and semi-automated. The application of these unit operating machines were size discriminating due to differences in length, width, and height. Thus, because the machines’ processing efficacies were not satisfactory for fish outside of the specified size range, they were culled and discarded. Therefore, mechanical unit operations were similar by efficiency with activities reported by sanitary engineering designers and machine developers (Figures 13-15).
De-scaling
De-skinning
De-boning
Results from iridium oxide based pH sensors for fish safety and quality controls
Freshness is an important aspect of food safety. Hence, overall food safety is the sum total of all the desired qualities that make food palatable. As a result, food quality control and monitoring are critical during transit and storage. According to the findings of this peer-reviewed paper, an Iridium oxide-based pH sensor for the assessment of fish safety and quality controls outperformed traditional glass electrodes in the monitoring of fish products. Despite the fact that conventional glass-type electrodes are commonly utilized, they nonetheless have some drawbacks in some applications. Due to the brittleness of glass, size constraints, and lack of deformability, the glass rod sensor structure is challenging to use for in vivo food monitoring applications. Therefore, Iridium oxide-based pH sensor can not only measure the quality and safety of fish products before the mechanical unit operation like de-scaling, de-skinning and deboning but over the wide range applications. Quality and safety in fish products can be measured using Iridium oxide-based pH sensor without the need of workers or practitioners starting from the point of capture and culture to advanced storage and tracking and trading via global supply chain manner. It was an instrument that brings traceability to the quality and safety of fish products worldwide (Figure 16).
A flexible IrOx pH electrode, RFID transponder (tag) circuit, and reader circuit are included in this RFID wireless pH sensor module. To achieve coupling efficiency, the sensing concept relies on inducting coupling between coil antennas (L) and capacitance (C) to generate the resonant frequency (Figure 17).
As a result, the flexible IrOx pH sensor detects the RFID Tag connecting oscillator as the frequency modulator's control mechanism while the data acquisition or spectrum analyzer system records data via the RFID Reader.
Hygienic design of fish processing equipment is more critical than ever before, and it is addressed in most regulatory and industry fish product safety programs. The phrases employed, however, are only loosely defined, and the interpretation of acceptability is left to the individual auditor and his or her aptitude for equipment evaluation. As we go forward with the implementation of fish product safety programs, we must pay more attention to the sanitary design aspects of equipment by developing more explicit and meaningful equipment requirements to assure compliance and fish product safety. Thus, the American Meat Institute and the Grocery manufacturers association, for example, have recently released recommendations that contain hygienic design principles. Therefore, the invention of indigenous and cutting-edge sensors, which are tough enough to resist the harsh manual operations and allow us to monitor fish processing, is a remarkable success. So, the entire instrumentation is constructed around these sensors, including the necessary electronics, new signal processors, and other peripherals for data storage, PC compatibility, and wireless transmission to remote locations, among other things. Furthermore, the information may be retained for a long time and retrieved since the instruments are intended to work with computers and memory modules.
Fish product safety and quality are frequently monitored with pH sensors. This is to overcome the three main mechanisms that cause fish to rot: enzyme autolysis, oxidation, and microbial growth. Therefore, before conducting any nutritional and processing parameters in the food industry's fish processing sector, it is necessary to manage temperature, chemical activities, enzymatic activities, and microbiological deterioration using pH sensors at the higher state of flexible IrOx pH electrode sensors. This is because fisheries require the most common processes used in the industry in the past, present, and future. Because pH is employed in so many chemical processes, the pH of almost all aqua samples is measured at some point. The most popular and latest iridium oxide pH sensor systems use amperometric or potentiometric devices. Hence, the most common potentiometric approach uses a glass electrode because of its superior selectivity for hydrogen ions in a solution, durability, and convenience of use. This is a crucial activity in the food industry because, prior to any processing mechanical unit operation, advanced pH measurement of fish safety and quality should be the primary application for the purpose of a high-quality product as the final end of the food industrial product development. Therefore, the industrially developed food product from fish should ensure containment and be convenient, communicable, and protective from any degradation to humans. As a result, it was the most effective approach for preventing fish and fish products from spoiling. In order to optimize the method, more research is needed to better understand the impact of proximal fish composition, post-harvest history, ambient conditions, and deliberate checkups of microbial levels in the food industry.
The pH values of fish products can be monitored both in culture and in capture fisheries before processing into different products in the food industry. After the harvesting of fish resources, pH testing should be the priority for the further mass and automated mechanical unit activities such as de-scaling, de-skinning, and de-boning operations for the assurance of safety and quality of the final product.
Before purchasing facilities, universities and food processing companies should focus on recruiting skilled practitioners or trained skilled laboratory technicians to advance food machinery installation and operation for the purpose of fish processing. As a result, this enables unit operations such as descaling, de-skinning, and de-boning will not be inefficient due to a lack of skilled labor for the operation. Gaping can thus be reduced by employing good handling techniques both above and below deck.
Cool the fish as soon as possible after catching them, and handle them with care at all times. Before handling any fish that has warmed, it should be chilled in cold sea water or ice.
Freeze fish as soon as possible after death; fish frozen before rigor develops will gape the least. This is particularly critical with haddock, which can be difficult to freeze without gaping.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [Pub Med]
[Crossref] [Google Scholar] [PubMed]
Journal of Bioprocessing & Biotechniques received 3351 citations as per Google Scholar report