Research Article - (2021) Volume 11, Issue 4
Received: 01-Mar-2021
Published:
25-Mar-2021
, DOI: 10.37421/2161-0525.21.11.629
Citation: Ughamba KT, Nnaji N, Ogbonna KE and Anyanwu
“Selling Points of Sewage Sludge as an Enhancing Agent of Bioremediation of
Diesel Oil-Polluted Soil.” J Environment Analytic Toxicol 11 (2021): 629.
Copyright: © 2021 Ughamba et al. This is an open-access article distributed
under the terms of the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.
Bioremediation employing the action of microbes alone has been shown to be inadequate. The aim of this study was to evaluate the efficacy of sewage sludge (SS) in enhancing bioremediation of diesel oil-polluted soil. Diesel oil was introduced into the soil at the concentration of 10 % (v/w) and mixed with 5%, 10% and 15% (w/w) of sewage sludge. The remediation of the oil was determined gravimetrically and spectrophotometrically using n-hexane as extractant. Effectiveness of the remediation strategy was assessed by the seed germination toxicity test At the end of forty-two days, 32.22 % oil loss was recorded in the unamended polluted soil while 58.33% oil loss was recorded in the soil amended with sewage sludge. Hydrocarbon- utilizing bacteria (HUB) counts were significantly high (P≤0.05) in the sewage sludge-amended options, ranging from 5.3 ±0.9 x 106 to 12.3±0.75 × 106CFU/g soil, as compared to the unamended control soil which gave 1.0 × 106- 3.8 × 106CFU/g of soil. The hydrocarbon- utilizing bacteria isolated from both the control and amended soils were identified tentatively as Bacillus cereus, Pseudomonas putida, Micrococcus varians, Corynebacterium spp and Staphylococcus spp based on their cultural, morphological and biochemical characteristics. The fungal counts in the SS-amendment options were also higher than was recorded in the control option ranging from 3.8 x 105 ± 0.2 to 11.6 x 105 ±0.25. Aerobic fungi isolated were Aspergillus niger, Aspergillus flavus, Fusarium sp, Cladosporium sp and Penicillium sp. The highest oil loss and germination indices were recorded in SS-amended options. There was a significant difference (P≤0.05) in oil loss and germination index between the unamended control soil and polluted soil amended with 15% SS.
Bacteria • Sewage sludge • Biostimulants • Diesel oil • Pollution
Sewage sludge has been widely published as a biostimulating tool in several biostimulation-based bioremediation of hydrocarbon-polluted soils. Specifically, few reports exist so far concerning the dynamics of sewage sludge and diesel oil remediation. In this work, sewage sludge tremendously enhanced bioremediation of diesel oil-polluted soil and the impact of sewage sludge was highly significant. The reason(s) for the observed trend was sequentially and systematically documented.
Petroleum-based products are the major energy source for vehicles, daily life and industry owing to their high energy content. Exploration, mining and transport of the same in developing countries have led to serious environmental hazards due to accidental spills (Ayandele, 2018) [1]. The increasing use of diesel in car engines, industrial trucks and generators has led to a marked increase in the demand for diesel fuel in Nigeria and damages due to soil contamination by diesel oil may be extensive and have long term effect (Ogbo, 2009) [2]. Release of hydrocarbons into the environment, whether accidentally or due to anthropogenic activities, is a main cause of water and soil pollution.
Diesel oil is a mixture of aromatic compounds and alkanes and is usually documented as soil pollutants because of their frequent release from accidental spills and leakage from storage tanks (Agarry and Latinwo, 2015). Diesel is a medium-weight petroleum fuel with a boiling point range of 1750C to 355 0C (Brady, 2001). It is composed of over 200 petroleum hydrocarbon compounds corresponding to the molecular weight range of C10-C28 alkanes. The exhaust fumes of diesel contains up to forty air pollutants including many suspected or known carcinogenic substances such as arsenic, formaldehyde and benzene. It also contains other harmful environmental pollutants, including nitrogen oxide, currently the single most important ozone-depleting emission (Awofeso, 2011) [2-5].
Oil exploration and exploitation are very lucrative and major revenue earner in Nigeria. However, like most industrial activities, it produces environmental hazards that are “slow poisons” in that they often take months and years to cause disease and death. The slow and obvious environmental hazards occasioned by exploration of oil and exploitation of the same make it difficult to fully understand their impart in the health of Nigeria as a people, especially in the oil-bearing communities, even with the emergence of non-communicable diseases as major causes of ill health in Nigeria.
The need for remediating diesel oil-polluted areas has induced development of new technologies to detoxify contaminants not only through chemical or physical methods which are expensive, but through biological techniques as well. Bioremediation is an eco-friendly and cost-effective option that removes contaminants or renders them innocuous using natural biological activity. Microorganisms degrade these compounds by using enzymes in their systems and can be useful in cleaning up contaminated sites (Abioye, 2009a) [6]. However, bioremediation employing the action of microbes alone has proven rather inadequate. Lack of essential nutrients such as nitrogen has been identified as a major limiting factor in petroleum hydrocarbon degradation among various factors. Therefore, the addition of organic or inorganic nutrients rich in nitrogen content remains an effective approach to enhance bioremediation process. The use of organic wastes to stimulate the indigenous hydrocarbon degraders has been widely demonstrated. However, few works have been done on the use of sewage sludge in enhancing the bioremediation of diesel oil-impacted soils. The present study was therefore undertaken to assess the enhancement potential of sewage sludge for diesel oil bioremediation in soil [7].
Collection of samples
Soil sample used in this study was collected from an agricultural farm land from different sites in Obukpa, Nsukka, Southeast, and Nigeria at a depth of 0-30 cm. The soil sample was air-dried for 48 hours and sieved through a 2-mm mesh. Sewage sludge was collected from the University of Nigeria sewage treatment plant while diesel oil was purchased from TOTAL filling station in Nsukka metropolis. Bean seeds (Phaseolus vulgaris) were obtained from the Department of Crop Science, University of Nigeria, and Nsukka.
Physicochemical analyses soil and sewage sludge
Physicochemical properties of soil and sewage sludge such as particle size distribution, percentage moisture content, pH, total organic carbon (%TOC), % nitrogen content and total phosphorus were analysed following standard protocol (APHA, 2008).Triplicate determinations were made for each assay [8].
Determination of extraction efficiency of different solvents for diesel oil
Three different organic solvents namely n-hexane, dichloromethane and diethylether were used to extract diesel oil and their extraction rates were determined. The best solvent in terms of extraction efficiency for diesel oil was later used for the bioremediation assay. The extraction efficiency was determined gravimetrically. Briefly, forty grammes of the soil sample was transferred into a 250 mL flask and polluted with 4 mL of diesel oil. A 4 mL quantity of diesel oil was used so as to simulate a 10% pollution condition that would be studied in the present work. A 100 mL quantity of the three organic solvents was added separately to each polluted soil sample set-up and the set-ups shaken for six hours at 180 rpm. The solution was then filtered using a Whatman No 4 filter paper and the weight of the extracted oil recorded. The extraction efficiency of the organic solvents for diesel was then determined by weight difference following the formula of Aremu et al. (2015). The experiment was carried out in triplicates [9].
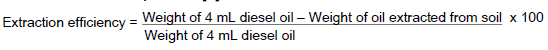
Soil preparation for bioremediation study
A 1 kg quantity of the sieved soil was placed in sterile polythene bags and 10 % (v/w) of diesel oil was added, mixed thoroughly, and left undisturbed for 48 hours. After the two days, 5%, 10% and 15% (w/w) pulverized sewage sludge were, respectively, introduced into the diesel oil-polluted soils and mixed thoroughly. Soil sample contaminated with 10% (v/w) diesel oil without sewage sludge amendment served as control. The moisture content of the soil was adjusted to 60% water holding capacity by the addition 50 mL of sterile distilled water (three times weekly) and the set-up kept at room temperature (28±2oC). The experiment was set up in triplicates.
Determination of diesel oil removal from soil
Periodic sampling from each polythene bag was carried out every seven days in order to determine the residual diesel oil. Gravimetric and spectrophotometric methods of Adesodun and Mbagwu (2008) with slight modification were employed in the determination of residual diesel oil present in both the control soil and amended options [10]. Composite soil samples weighing five grammes were put in a 50 mL flask and 10 mL of n-hexane was added. N-hexane was used because it extracted the highest amount of oil in the extraction efficiency experiment (see section 3.2). The set-ups were shaken with a rotary shaker at 180 rpm for 10 hours to allow for an efficient and complete oil extraction with n-hexane. The mixture was then filtered with a whatman No 4 filter paper. The filtration was done repeatedly two times to ensure complete extraction of the liquid phase. The filtrate was diluted by adding 50 mL of n-hexane to 1 mL of the extracted diesel oil and the absorbance of the solution measured at 460 nm (Shimadzu UV 1800) using n-hexane as blank. The total petroleum hydrocarbon (TPH) was estimated by extrapolating from a standard curve derived from different concentrations of fresh diesel oil diluted with n-hexane. Percent remediation (R) was cal culated using the following formula:

Where TPHr and TPHi are residual and initial TPH concentrations
Enumeration and identification of oil-utilizing bacteria and fungi
Ten-fold serial dilutions of soil samples from each option was made by suspending 10 g of soil in 90 mL of distilled water and shaken vigorously for proper mixing. The suspension was serially diluted up to 10-8. A 0.1mL aliquot of 10-5, 10-6and 10-7 dilutions was separately inoculated in sterile nutrient agar plates by the standard spread plate method (Odokuma and Ibor 2002) for active aerobic heterotrophic bacteria isolation [11]. The nutrient agar medium was supplemented with 50 μg/mL nystatin to suppress the growth of fungi. The agar plates were incubated at 37°C for 24 h after which colony forming units per gram of soil samples were calculated. Three replicate samples from each oil-polluted soil were withdrawn every 7 days for the enumeration of total aerobic heterotrophic bacteria (AHB).
Hydrocarbon utilizing bacteria (HUB) in the soil samples were enumerated on Bushnell Has agar using the vapour phase transfer method as described by Nwogu et al. (2015): A filter paper saturated with sterile diesel oil was aseptically placed on the inside of the cover of inverted inoculated petri dishes and incubated at 28°C for 7 days [12]. Morphologically different colonies of hydrocarbon-utilizing bacteria were picked and pure isolates obtained by repeated sub-culturing on nutrient agar. The bacterial isolates were characterized and tentatively identified using microscopic techniques and biochemical tests such as catalase, urease, oxidase, starch hydrolysis, spore forming, H2S production, motility, citrate utilization and methyl-red.
For the enumeration and isolation of fungi, 0.1ml of the appropriate dilution of each of the set-ups was inoculated into Sabouraud Dextrose Agar (SDA) plates and incubated at 28 ± 2o C for 4 days. Colony counts were taken and pure isolates obtained by repeated sub-culturing on SDA plates. The fungal isolates were characterized by slide culture and microscopic techniques and identified by the schemes of Tsuneo (2002) [13,7].
Seed germination toxicity test
Toxicity of the soil to seed germination after a 42-day bioremediation experiment was assessed following the seed germination test of Jaqueline et al. (2013) [5]. Seeds of Phaseolus vulgaris (common bean) were used in this study owing to their sensitivity to hydrocarbon in soil. For each soil preparation, 40 g of thoroughly-mixed remediated soil samples from both the control soils and the amended soil was placed in 100 × 15 mm petri-dish. Six viable bean seeds were placed evenly throughout each petridish and covered with 10 g of dry sand. The moisture content of the set-ups was maintained at 60% water holding capacity. Triplicate determinations were made for each assay. At the end of 10 days, the number of seeds that germinated from the surface of the soil was counted and root length measured to the nearest centimeter using a metre rule. The results were evaluated using the formula of Millioli et al. (2009) with slight modification. Soil neither polluted nor amended served as the positive control soil while polluted soil without amendment served as negative control [14].
• Germination index (%) = (SG×LR)/100
• SG=(ET/CG) × 100
• LR=(LRT/LRC) ×100
Where SG= number of seed germination, LR=root length (elongation), ET=number of seeds that germinated on treated soil, CG=number of seeds that germinated on positive control soil, LRT = root length on treated soil, LRC=root length on positive control soil.
Statistical analysis of data
The data obtained in the present study were subjected to analysis of variance (ANOVA). Relationship between variables and comparison of means of the different treatments were tested for level of significances at P≤0.05 using least square difference and post-hoc multiple comparison tests. The data analysis was performed using SPSS.
Physicochemical properties of soil and sewage sludge
The physicochemical properties of the soil and SS used in this study are presented in Table 1. The % organic nitrogen content of the soil was 0.02 and available phosphorus content of 10.64%. Other parameters of the soil in percentage are: TOC 2.49, moisture 15.38 and the pH is 4.9. The sewage sludge had pH, nitrogen content, TOC and total phosphorus content of 3.4±0.015, 0.28±0.03, 11.97±0.07 and 17.920.034, respectively and nitrogen content of 0.28 and TOC of 11.97. Pig droppings had nitrogen content of 0.238 while poultry manure had organic nitrogen content of 0.098 and TOC of 56.86.
| Parameter | Non-polluted Soil | Sewage sludge |
|---|---|---|
| pH | 4.90±0.37 | 3.40±0.015 |
| Nitrogen | 0.042±0.02 | 0.28±0.03 |
| Organic carbon | 2.49±0.45 | 11.97±0.07 |
| Phosphorus (PPM) | 10.64±0.5 | 17.92±0.034 |
| Moisture(%) | 10.38±0.3 | 8.69±0.229 |
| Clay (%) | 71.00 ± 0.41 | - |
| Silt (%) | 19.50± 0.06 | - |
| Sand (%) | 9.50± 0.08 | - |
| Texture | Clayey Loam |
Table 1: Physicochemical Properties of Soil and Sewage Sludge
Extraction efficiency of solvents for diesel oil
The amount of diesel oil (in percentage) that was extracted by three different solvents namely: n-hexane, dichloromethane and diethylether six hours after polluting soil with 10% (v/w) diesel oil were 80.72%, 80.68%, 80.56%.
Bioremediation of diesel oil in soil
The levels of bioremediation of diesel oil in the unamended control soil and soil amended with 5% SS are shown in Figure 1. Percentage oil loss in the sewage sludge-amended option ranged between 27.78 ± 1.27 and 58.33 ± 0.67. In the unamended control soil, the percentage loss ranged between 19.93 ± 0.96 and 32.22 ± 0.17.
Figure 1: Bioremediation of diesel oil in polluted soil amended with 5% SS.
Figure 2 shows the level of oil loss in control soil and polluted soil amended with 10% SS over a 42-day period. Percentage oil loss in the SS-amended option ranged from 35.94±0.98 to 48.75±1.27
Figure 2: Bioremediation of diesel oil in pollute d soil amended with 10% SS.
The level of oil loss in the control soil and polluted soil amended with 15% SS over a 42-day period is presented. Percentage oil loss in the SS-amended option ranged from 36.61 ± 1.09 to 58.33±2.12
Active aerobic heterotrophic bacterial (AHB) counts
The total heterotrophic bacteria in control soil and polluted soil amended with 5% SS are presented. AHB counts in the SS-amended option ranged from 8.3 x 107 ± 2.78 to 22.5 x 107± 0.71 CFU/g. Control soil had AHB counts ranging from 1.0 x 107 ± 0.6 to 20 x 107 ± 0.9
AHB counts in the control option and polluted soil amended with 15% SS. AHB counts in the SS-amended soil ranged between 10.5±2.08 x 107 and 24.0 x 107±0.78.
Hydrocarbon-Utilising Bacterial (HUB) counts
The profile of HUB count in control soil and oil-polluted soil amended with 5% SS over a 42-day period. HUB counts in the SS-amended option ranged from 4.8 x 106± 0.71 to 11.0 x 106± 0.78. The control option had a HUB count ranging from 0.8 x 106 ± 0.13 to 3.2 x 106 ± 0.27.
The hydrocarbon-utilising bacterial load in the control option and oilpolluted soil amended with 15% SS within 42 days. while 6.2-12.2 x 106 ±0.31-0.48 are the counts recorded in the SS-amended option.
The hydrocarbon-utilising bacterial load in the control option and oilpolluted soil amended with 15% SS within 42 days. while 6.2-12.2 x106 ±0.31-0.48 are the counts recorded in the SS-amended option.
Identities of bacterial isolates
The microscopic and biochemical characteristics of the isolated hydrocarbon-utilising bacteria. The HUBs are identified tentatively as Bacillus licheniformis, Pseudomonas putida, Corynebacterium sp., Micrococcus varians, Staphylococcus aureus and Bacillus cereus
Fungal counts
The active aerobic heterotrophic fungal counts in all the 5% amendment options and unamended control soil over a 42-day period. Fungal counts in the SS-amended soil ranged from 3.8 x 105 ± 0.11 to 9.6 x 105 ±0.38 while 0.3- 2.0 x 105 ±0.13-0.23 is the range of fungal counts recorded in the unamended control soil.
Fungal counts in the oil-polluted soil amended with 10% SS and the control option over a 42-day period. Fungal counts ranged from 4.9 x 105 to 10.9 x 105 in the SS-amended soil.
Fungal load recorded in the 15% SS-amendment options over a 42-day period. It ranged from 6.0 x 105 ±0.22 to 11.6 x 105 ±0.39
Identities of fungal isolates
The cultural and microscopic characteristics of the isolated hydrocarbonutilising fungi. Fungi isolated predominantly were identified tentatively as Aspergillus niger, Aspergillus flavus, Fusarium sp., Cladosporium sp. and Penicillium sp.
Seed germination toxicity
Seed germination parametres employed in this study. SG (%) ranged from 66.7 to 83.3 across all amendment levels in the SS-amendment option while negative and positive control soils had SG (%) of 16.7 and 100, respectively. LR (%) ranged from 58.5 to 87.7 across all amendment levels in the amendment option while 16.9 and 100 were recorded in the negative and positive control, respectively. Furthermore, GI ranged from 39.0 to 73.1 across all amendment options. Negative and positive control had GI of 2.8 and 100
Several studies on hydrocarbon remediation have shown that bioremediation employing the activities of microbes alone proved inadequate (Akpe, 2015; Onuoha, 2013; Agamuthu and Dadrasnia, 2013). Ubalua (2011) argued that while hydrocarbons are excellent sources of carbon and energy to microbes, they are incomplete foods in that they do not contain significant concentrations of other nutrients such as nitrogen and phosphorus required for microbial growth. The dearth of nutrients in soils polluted with hydrocarbons poses a challenge to bioremediation; nevertheless, nutrient addition generally favours soil hydrocarbon-utilising bacteria and fungi, ultimately resulting in enhanced bioremediation of hydrocarbon-polluted environment (Abioye et al., 2009a). By adding organic wastes, the C:N and C:P ratios of the soil becomes closer to the bacterial requirements of the same.
It was reported by Agarry and Latinwo (2015) that bioremediation of diesel fuel depended on phosphorus ability. However, Agarry and Jimoda (2013) argued that some nutrient sources might supply enough phosphorus to restore the microbial C:P relationship but become unavailable due to their low solubility. Pivotal, therefore is the knowledge of nutrient bioavailability to planning an efficient bioremediation protocol.
The percentage nitrogen content of the soil used in this study was comparably low (Table 1). Adesina et al. (2011) argued that hydrocarbon degraders need more nitrogen and phosphorus than is normally present in soils to convert excess carbon present in hydrocarbon pollutants to biomass. In a similar vein, Thapa et al. (2012) argued that although microorganisms are present in contaminated soil, they cannot necessarily be present at levels required for bioremediation of the site, hence the need for their growth and activities to be stimulated. The soil used in this study had high C:N ratio of 59:1 (Table 1). Furthermore, pollution of the experimental soil with 10% v/w diesel oil introduced more carbon to the soil, thereby increasing its C:N ratio. As a general rule of thumb, materials with a C:N ratio greater than 25:1 stimulate immobilization (Robertson and Groffman, 2007). Carbon is the most basic form of nutrient required for living organism. In addition to this, bacteria also need macronutrients such as nitrogen and phosphorus to ensure effective degradation of the oil. However, the ratio of carbon to essential nutrients such as nitrogen and phosphorus is critical to the realization of an effective bioremediation. As organisms convert excess carbon present in hydrocarbon spills onto biomass, they require corresponding proportions of these essential nutrients. The optimum nutrient balance required for hydrocarbon remediation is C:N:P equals 100:10:1(Okoh, 2006;Thapa et al., 2012) [3]. Teng et al., (2010) recorded higher polycyclic aromatic hydrocarbon degradation in soil amendment with C:N ratio of 10:1 than those with C:N ratio of 25:1 and 40:1, respectively [8]. In the present study, therefore, the addition of sewage sludge rich in organic nitrogen obviously balanced the C:N ratio of the soil at a level that enhanced growth and activities of microbes. This enhancement ultimately favoured degradation of diesel oil pollutant [14-16].
The result of the extraction efficiency experiment clearly indicated that n-hexane was the best choice in extracting diesel oil under the conditions employed in this study. This is due to the fact that the highest amount of diesel oil was extracted with n-hexane among other solvents such as dichloromethane and diethylether used in this study.
Percentage oil loss (bioremediation) increased appreciably from the first week to the sixth week in both the amendment and control options. However, as was observed throughout the study period, oil loss increased with increasing concentration of sewage sludge. Highest oil loss was noted in the polluted soil amended with 15% SS. The observation of highest oil loss in the SS-amended option was probably due to its relatively higher content of organic nitrogen (Table 1). Positive effects of nitrogen amendment have been demonstrated (Margesin et al., 2007 2001). It has been reported that when oil is applied at rates of 0.5-10% based on the weight, extensive bioremediation of the oil components occurs within the first three months (Agamuthu and Dadrasnia, 2013). Oil loss in the control option also increased notably from 19.23% to 32.22% at the end of 42 days. Dadrasnia and Agamuthu (2010) in a similar study on diesel oil remediation using soy cake, potato skin and tea leaf amendment recorded 35% oil loss in the control option. Nwogu et al.(2015) in a 42-day study on crude oil remediation using only goat manure as amendment recorded 8.15% oil loss for the control microcosm at the end of the study. Part of the oil loss in the control option could be due to some factors such as natural bioattenuation by the indigenous hydrocarbon-degrading flora, photodegradation, volatilization, sorption, dilution and dispersion. Similar observation was noted by Agarry and Jimoda (2013). Greater oil loss was recorded in the amendment options than the control option. Similar trend have been widely documented (Adesodun and Mbagwu, 2008; Agamuthu and Dadrasnia, 2013; Agarry and Jimoda, 2013) [15,7-12].
Even though the application of biostimulation strategy in bioremediation has gained wide acceptance, reports on the biostimulation potentials of organic amendments have been widely divergent in literature. While some researchers documented a direct (linear) relationship between hydrocarbon remediation and impact of organic wastes (Abioye et al., 2009a; Stephen and Temola, 2014), another proved otherwise by reporting that natural attenuation was more successful than biostimulation in Hong Kong soil (Bento, 2005). It was also found that nutrient supplementation had no significant effect on the remediation of polluted soils (Seklemova et al., 2001). However, it was asserted that different soils have varying inherent microbial potentials to degrade hydrocarbons (Padayachee and Lin, 2011). Ways to activate these potentials must bring into account that most degradation potentials are widely distributed among microorganisms and indigenous microbes are always present in small numbers [16].
The growth and activities of heterotrophic bacteria and fungi is a biological
indicator of the impact of organic wastes. In the present study, AHB counts
increased progressively throughout the study in the amendment and control
options. However, AHB counts were higher in the amended option (at all
amendment levels) than the control option. It was also observed that highest
counts were recorded at 15% amendment level. This could be as a result of
enhanced nutrient level in the highest level of amendment. Earlier researchers
noted similar observation (Onuoha, 2013; Stephen et al., 2013). Stephen et al.,
(2013) in a study on diesel oil remediation using cowpea chaff recorded AHB
counts of 8.0 x 106-30.0 x 106 in the amended option. Nwogu et al.(2015) in
a similar study recorded AHB counts ranging from 3.4 x 103-2.9 x 105CFU/g
in the control soil. Similarly, HUB counts increased markedly from 4.8 x 106 ±0.9 to 12.3 × 106 ±0.75 CFU/g soil throughout the study period and higher
HUB counts were recorded in the amended option than the control. Similar
observation had been documented (Ibiene et al., 2011). Furthermore, as
was noted in the population count profile of AHB and HUB, fungal population
increased progressively throughout the study period and was highest at the
highest amendment level (15%). The observation of greater AHB, HUB and
fungi in the amended option than the control option might also be attributed to
the fact that sewage sludge harbor great diversities of microbes with inherent
hydrocarbon-degrading potentials. Technically speaking, therefore, addition
of organic wastes such as sewage sludge can be regarded as ‘uncontrolled
bioaugmentation’. It was noted in this study that AHB counts were higher in
number than their hydrocarbon-utilising counterparts. Akpe et al.(2015) noted
a similar trend. It stands to reason therefore that HUB is a significant proportion
of heterotrophic bacteria that evolved probably as a result of incessant soil
pollution with hydrocarbons. The HUB isolated in this study were identified
tentatively as Bacillus licheniformis, Pseudomonas putida, Corynebacterium
sp., Micrococcus varians, Staphylococcus aureus and Bacillus cereus . These
bacteria have been widely reported (Onuoha, 2013; Akpe et al., 2015; Stephen
et al., 2013) as having hydrocarbon-utilisation attributes. Also, fungi isolated
in the present study were identified tentatively as Aspergillus niger, Aspergillus The growth and activities of heterotrophic bacteria and fungi is a biological
indicator of the impact of organic wastes. In the present study, AHB counts
increased progressively throughout the study in the amendment and control
options. However, AHB counts were higher in the amended option (at all
amendment levels) than the control option. It was also observed that highest
counts were recorded at 15% amendment level. This could be as a result of
enhanced nutrient level in the highest level of amendment. Earlier researchers
noted similar observation (Onuoha, 2013; Stephen et al., 2013). Stephen et al.,
(2013) in a study on diesel oil remediation using cowpea chaff recorded AHB
counts of 8.0 x 106-30.0 x 106 in the amended option. Nwogu et al.(2015) in
a similar study recorded AHB counts ranging from 3.4 x 103-2.9 x 105CFU/g
in the control soil. Similarly, HUB counts increased markedly from 4.8 x 10
Seed germination toxicity test has been demonstrated as a good parameter of assessing the efficacy of bioremediation on contaminated soils (Jaqueline et al., 2013). The highest germination index was recorded in the SS-amended option at 15% amendment level . GI recorded in the present study followed the same pattern as bioremediation results and microbial counts. All the seeds germinated in the positive control soil while one seed germinated in the negative control soil. This could be attributed to absence of oil pollution in the positive control soil and oil pollution in the negative control soil. Oil pollution has been identified by different researchers (Okechalu et al., 2014; Ogbo, 2014) as having adverse effects on plant development parametres. Agbogidi and Ilondu (2013) recorded growth of all seeds of Moringa olifera planted in the positive control option. Okechalu et al. (2014) also reported 99.6% germination in the positive control option. Phaseolus vulgaris normally germinates within 8-10 days but the germination was delayed to 19-21 days owing to slightly heavy pollution simulated in this study (10%) which had not been fully remediated (58.33%) as at the end of the 42-day study period [10,14].
There was a significant difference in bioremediation level and microbial counts (P≤0.05) between SS-amended soil and control even at 5% level.
This study showed that bioremediation employing the impact of organic amendments to soil polluted with diesel oil enhanced the activities of the remediating microflora by improving nutrient supply. Sewage sludge alone or in combination with other wastes can therefore offer a good alternative to the rather expensive physical and chemical methods of hydrocarbon remediation.
The authors’ appreciation goes to Dr. V.N. Chigor of the Department of Microbiology, University of Nigeria for the use of his laboratory facilities.
The authors declare that no conflict of interest exists.
Environmental & Analytical Toxicology received 6818 citations as per Google Scholar report