Research Article - (2024) Volume 14, Issue 1
Received: 01-Jan-2024, Manuscript No. jbpbt-23-119998;
Editor assigned: 03-Jan-2024, Pre QC No. P-119998;
Reviewed: 15-Jan-2024, QC No. Q-119998;
Revised: 22-Jan-2024, Manuscript No. R-119998;
Published:
30-Jan-2024
, DOI: 10.37421/2155-9821.2024.14.601
Citation: Poulain, Benjamin, Hassana Hsein, Pierre Tchreloff and
Claudia Nioi. “Effects of Different Drying Methods on the Antioxidant Properties
of White Wine Lees.” J Bioprocess Biotech 14 (2024): 601.
Copyright: © 2024 Poulain B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This study investigated the influence of different drying processes and their operating conditions on the preservation of the antioxidant properties of white wine lees. Four white wine lees were dried using different methods: Spray Drying (SD), Freeze Drying (FD) and Vacuum Oven (VO). Physicochemical characteristics of the dried lees powders were analysed in terms of residual moisture content, particle morphology, particle size and antioxidant properties which were assessed via DPPH, FRAP assays and Total Polyphenol Content (TPC). The powders obtained via the different drying processes had different physical characteristics, with particle sizes ranging from 5 to 100 μm depending on the drying technic. FD and VO resulted in larger particle sizes than the powders obtained from SD. This result was confirmed by SEM observation and laser diffraction particle sizing technique. After FD, VO and SD<200°C the antioxidant capacities of the all studied lees was maintained. The DPPH values ranged from to 9.5 to 10.8 mg TE/g DM and the FRAP values ranged from 31.2 to 37.3 mg TE/g DM. Even after 12 months of storage at room temperature, the antioxidant properties of the wine lees remained unchanged. It can be concluded that these drying techniques make it possible to preserve the antioxidant properties of white wine lees and thus that wine lees are a potential natural source of antioxidants for food, nutraceutical, pharmaceutical and cosmetic fields.
Wine lees • Drying methods • Spray drying • Freeze drying • Vacuum oven • Antioxidant activity
FD: Freeze-Drying; GSH: Glutathione; MC: Moisture Content; SD: Spray-Drying; SEM: Scanning Electron Microscopy; TE: Trolox Equivalent; TPC: Total Polyphenol Content; VO: Vacuum Oven; WL: Wine Lees
Wine Lees (WL) can be defined as the residue found in wine containers after fermentation, storage or winemaking stabilisation treatments [1]. WL represents 6-10% (w/w) of the total weight of grapes and is the second most important by-product of winemaking after grape pomace (representing 20- 25% of total weight of the grapes used for winemaking). They are composed of both solid and liquid fractions. The solid fraction mainly comprises yeasts, organic acids, carbohydrates, inorganic salts, proteins, peptides and phenolic compounds. The liquid fraction is rich in ethanol and organic acids [2,3]. The composition of WL is influenced by many different factors, such as grape variety, land type and vineyards practices, as well as winemaking conditions (fermentation and aging).
Over the last 10 years, interest in converting this bio-waste into a valueadded product has increased and several studies have shown the potential for valorization of the WL solid fraction [4]. A useful application of WL is the production of biodegradable polymers (such as PHBH, PHBV and PBS) for developing food packaging [5]. WL polysaccharides (mannoproteins from yeast biomass) have been used as winemaking additives to increase wine chemical stability [6-8]. The polyphenols fraction of WL has been widely studied with a focus on ensuring or improving the qualitative characteristics of food, such as wine and ice cream. In addition, different polyphenol compounds, like non-flavonoids and flavonoids, were quantified in WL and their concentration was found to be related to WL antioxidant capacity. Recently, squalene, an antioxidant compound, was detected in and recovered from lees [9,10]. Finally, during white winemaking, wines aged in contact with lees for 3-10 months exhibited greater resistance to oxidation (colour and aroma) than wines aged without lees. This seems to be related to their capacity to preserve glutathione (GSH), an antioxidant tripeptide present in grape and produced by yeasts during alcoholic fermentation [11].
While the antioxidant capacity of red WL has been proved to be related to polyphenols, the compounds involved in the reducing power of white WL are still unknown [12]. Although the role of glutathione has been demonstrated [7], recent studies have shown that S- and N-containing compounds are the main contributors to the antioxidant power of wine, especially after wine has been aged in contact with lees [13]. Thus, when studying the antioxidant potential of WL, the challenge lies in identifying new antioxidant compounds and in assessing the potential of the WL as a useful source of new antioxidant compounds [14,15]. In a valorization strategy, the drying process is considered as the first step in the extraction of potentially useful antioxidant compounds from WL. Drying biomass reduces storage and transportation costs, but the preservation of bioactive compounds and how they are managed depend highly on the operating conditions of the drying method applied. Several studies have investigated the effects of different drying methods on the chemical changes of compounds in herbs, fruits and by-products and on the bioactivity of these compounds [16-20]. In general, a reduction in compound bioactivity occurs when drying temperatures are higher than 50°C-150°C [21-23].
Lyophilization or Freeze-Drying (FD) - the most commonly used drying process for pharmaceutical compounds – has low impact on the bioactivity of these compounds [24]. Freeze-drying is a complex process that involves many steps. It consists in freezing and then drying under vacuum; i.e., initial freezing followed by primary (ice sublimation) and secondary drying steps. Primary drying is the most time- and energy-consuming stage of lyophilization and it therefore considerably contributes to the total operational cost [25].
Another drying process is atomisation or Spray Drying (SD), a widely used technique in the food industry which consists in the transformation of liquid products to powdered products by exposing liquid droplets to a current of hot air under pressure. This drying process is usually carried out to ensure microbiological stability and to avoid any risk of food products biologically degrading. However, atomisation can result in problems related to the deposition or adhesion of particles, which reduces the process yield [17]. The physico-chemical properties and bioactivity of the obtained powders depend on the operating conditions (e.g., concentration of feed, temperature and pressure) and process optimisation is essential for preserving bioactivity while ensuring process yield.
The Vacuum Oven drying process (VO) is also a commonly used method for reducing moisture from raw matter like fruits, vegetables and medicinal plants [19]. It has the advantages of relatively low operational costs and being able to carry out drying at low temperatures. However, this method has several limitations: it has low energy efficiency and may not necessarily always produce dried products with a high retention of bioactive compounds [26]. In addition, being a surface heating method, it is characterized by a low drying rate: after the water has evaporated from the surface of the matrix, the remaining moisture is subsequently removed from the inner areas of the raw material driven by a moisture concentration gradient. This diffusion of moisture from the middle of the material to the surface is thus slow and rate limiting.
The present study aimed to evaluate the influence of these three drying methods - freeze drying, spray drying and vacuum oven drying - on the physicochemical properties and antioxidant activity of white wine lees powders. All the obtained powders were first analysed for moisture content, particle morphology and granulometry, then they were analysed in order to evaluate antiradical activity (via DPPH), antioxidant capacity (via ferric reducing power; FRAP) and Total Polyphenol Content (TPC).
Chemicals and materials
Ultrapure water (Purite Type I Ultrapure Water System, Veolia, France), HPLC grade ethanol (from VWR International, France) were used for sample preparation. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid (Trolox), iron (III) chloride hexahydrate, sodium acetate, glacial acetic acid and gallic acid were purchased from ThermoFisher Scientific (France). 2,4,6-Tri(2-Pyridyl)-s-Triazine (TPTZ), Folin-Ciocalteu reagent, hydrochloric acid were purchased from Sigma-Aldrich (France). Sodium carbonate was purchased from VWR (France).
Four wine lees samples were recovered from different white wines: two from a Sauvignon Blanc variety (Lees 1 and Lees 2) and two from a Semillon variety (Lees 3 and Lees 4) from the 2021 vintage (Sauterne, France). Before lees recuperation, the winemaking process comprised the following steps: destemming and pressing of the grapes, racking of the musts with turbidity adjusted to 220 NTU and fermentation with the addition of Saccharomyces cerevisiae yeast (Zymaflore® ST, Biolaffort France). When fermentation had started, the musts were transfer in oak barrels. Once the fermentations were completed, the wines were kept on their lees for 4 months, with regular stirring of the lees for the first 3 months and then they were racked. All the wine lees (samples 1, 2, 3 and 4) were collected from the bottom of the oak barrels and then dried applying the different processes.
Drying methods
Three different drying methods were assessed in this study: freeze drying, spray drying and vacuum oven drying. Before drying, all samples underwent a sieving treatment (500 μm mesh size) in order to eliminate all the exogenous residues in the wine lees, like wood or grape pomace. After drying, all the lees powders were hermetically stored in glass vials in dry air; the vials were stored at room temperature and protected from the light until analysis.
Freeze-drying: Freeze-Drying (FD) was performed using a Cryotec® compact pilot freeze-dryer (Montpellier, France). The samples were placed in trays for bulk lyophilisation. Three FD cycles with differing conditions were carried out in order to study the influence of the freezing step (freezing temperature and rate) and the drying steps (shelf temperature and duration) on the antioxidant properties of freeze-dried wine lees. Freezing was either performed by cooling shelves to -50°C, with a cooling rate of -1°C.min-1 and held at -50°C for 2 h (slow cooling rate), or by placing trays directly in a freezer at -80°C for 2 h (fast cooling rate). The primary drying step was performed by heating the shelves to -30°C or 20°C at a heating rate of +1°C.min-1. The chamber pressure was then decreased to 0.1 mbar for all FD cycles to assure primary drying (sublimation). The secondary drying step was carried out at a shelf temperature of 25°C and under a chamber pressure of 0.05 mbar for the cycles during which primary drying was carried out at -30°C. A summary of these process conditions is presented in Table 1.
| Cycle Name | Freezing Temperature | Primary Drying Temperature and Pressure | Secondary Drying Temperature and Pressure |
|---|---|---|---|
| FD1 | -50°C | -30°C; 0.1 mbar | 25°C; 0.05 mbar |
| FD2 | -50°C | 20°C; 0.1 mbar | 20°C; 0.1 mbar |
| FD3 | -80°C | -30°C; 0.1 mbar | 25°C; 0.05 mbar |
Spray drying: Spray Drying (SD) was performed using a BUCHI Mini Spray Dryer B-191 (Flawil, Switzerland); this is a co-current drying system comprising a two-fluid nozzle mechanism. The nozzle size was 0.8 μm. All wine lees suspensions were diluted to 5% (w/w) to prevent clogging of the nozzle. Spray drying was performed at three different inlet temperatures (120°C, 160°C and 200°C) in order to study the influence of the drying temperature on the antioxidant properties of spray-dried wine lees. The following operating parameters were set for all the SD batches: pump rate (8 mL.min-1), spray gas flow rate (50 mmHg, 700 m3.h-1) and the aspiration were maintained at 100%. Outlet temperatures were recorded for every batch and were 65, 95 and 130°C for inlet temperatures of 120, 160 and 200°C respectively. All SD powders were subsequently collected in a glovebox under dry air and hermetically stored in glass vials before analysis.
Vacuum oven drying: Wine lees distributed in trays were placed in a vacuum dry oven (Lab-line Instruments, USA) set at 50°C and 1 Pa. Drying in a vacuum oven allows solvents to evaporate more quickly and at a lower temperature than drying at atmospheric pressure. Moreover, drying is carried out in the absence of oxygen which can have deleterious effects on antioxidant properties. The collected samples had a very hard texture and looked like small pebbles. They were ground to powder using a ball mill (Retsch Mixer Mill MM 200, Haan, Germany) for 3 min at 70 vibration/oscillation amplitude.
Yield
Yield was calculated for all the drying processes as follows:
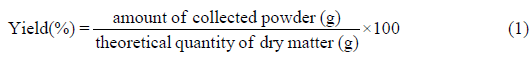
The theoretical quantity of dry matter was estimated by drying fresh lees in an oven at 105°C for 24 h. The mean value obtained was about 18 ± 2% (w/w).
Physicochemical properties of dried lees powders
Residual moisture content: The Moisture Content (MC) of the dried powders was determined using the Karl Fischer titration method with a titrator compact 20S (Mettler Toledo, Greifensee, Switzerland). About 20 mg of each powder was put into the combicoulomat fritless Karl Fischer/ HYDRANAL™ - Composite 5 Honeywell Fluka™ reagent and stirred for 2 min. The amount of water in the samples was then recorded.
Evaluation of particle morphology and particle size: Particle morphology was studied by Scanning Electron Microscopy (SEM) using the analyse mode (Hitachi TM3000, Japan). Samples were directly fixed to aluminum stubs with double-sided carbon tape.
The particle size (defined as diameter of particle) distribution was determined using the laser diffraction particle size technique. To measure particles in the dry state, the Aero S module was used with the following parameters: 2.5 bars and 30% of vibration (Mastersizer 3000, Malvern Panalytical, United Kingdom). To measure particles after dispersion in water, the Hydro 2000SM wet sample dispersion unit was used (Mastersizer 2000, Malvern Panalytical, United Kingdom).
Determination of antioxidant activity
DPPH radical-scavenging activity: The DPPH radical scavenging activity of each dry lees was carried out using the method of Brand-Williams W, et al. [27] with some modifications in order to fit in 96-well microplates [14]. The DPPH• Solution in ethanol (9.0 × 10-5 M) was prepared daily and 1.9 mL of this reagent was mixed with 0.1 mL of standard or rehydrated dry lees at 5 g.L-1. A blank sample containing 0.1 mL of EtOH/H20 (50:50, v/v) was prepared. After 30 min of incubation in the dark at 25°C, the samples were centrifugated at 12000 RPM for 2 min. Immediately after centrifugation, 300 μL of solution (sample and DPPH solution mix) was put on a 96-well microplate (four times for each sample). The absorbance was measured at 515 nm with microplate spectrophotometer UV-Vis MultiSkan Sky High (Thermo Fisher Scientific Inc., Waltman, MA, USA) and all analyses were carried out in triplicate (n=12). A calibration curve was obtained using Trolox concentrations from 0 to 125 mg.L-1 in EtOH/H20 (50:50, v/v) and the results were expressed as mg Trolox Equivalent per g of lees Dry Matter (mg TE.g DM-1).
Ferric Reducing Antioxidant Potential (FRAP) assay: The ferric reducing power of dry lees was determined using the method of Benzie IFF and Strain JJ [28], with some modifications, as described by González- Centeno MR, et al. [29], to adapt the method to 96-well microplates. The FRAP reagent was prepared daily by mixing 1 vol of 0.01 M TPTZ solution (in 0.04 M HCl) with 1 vol of 0.02 M FeCl36H20 aqueous solution and 10 vol of 0.3 M acetate buffer (pH of 3.6). All the solutions were used on the day they were prepared, except for the acetate buffer and hydrochloric solution. The reaction was carried out by adding 0.1 mL of standard or rehydrated dry lees at 5 g.L-1 to 1.9 mL of FRAP reagent, or 0.1 mL of EtOH/H20 (50:50, v/v) for a blank sample. After 30 min of incubation in the dark at 25°C, the samples were centrifugated at 12000 RPM for 2 min. Immediately after centrifugation, 300 μL of solution (sample and FRAP solution mix) was put on a 96-well microplate (n=4) and then the absorbance was measured at 593 nm. All the samples were analysed in triplicate (4 replicates on 3 different plates, n=12). Trolox was used for the calibration curve at concentrations of 0 to 125 mg.L-1 by using EtOH/ H20 (50:50, v/v) as the solvent and the results were expressed as mg Trolox Equivalent per g of lees Dry Matter (mg TE.g DM-1).
Determination of total phenolic content: Total Phenolic Content (TPC) was determined following the Folin-Ciocalteu method [30]. A volume of 0.2 mL standard or rehydrated dry lees at a concentration of 5 g.L-1 and 0.8 mL sodium carbonate (7.5% w/v) was added to 1 mL of Folin-Ciocalteu reagent (previously diluted 10-fold in deionised water). After 30 min of incubation in the dark at 25°C, the samples were centrifugated at 12000 RPM for 2 min at room temperature. After centrifugation, 200 μL of solution (all TPC reagents and samples) were put on a 96-well microplate (n=4) and absorbance was measured at 760 nm. All measurements were carried out in triplicate (n=12). Gallic acid (0 to 200 mg.L-1) was used as a standard for the calibration curve and Total Phenolic Content (TPC) was expressed as milligrams equivalent of gallic acid per g of lees dry matter (mg GAE.g DM-1).
Statistical analysis
The variability of the antioxidant properties (DPPH and FRAP) and the TPC of the wine lees after being subject to different drying methods was analysed using R software. The different effects of the experimental treatments on each lees were tested using analysis of variance (ANOVA) of the linear model Yi=m+DMi+εi, where DM is the drying method comprising eight levels of I (FL, FD1, FD2, FD3, SD120, SD160, SD200, VO) and εi is the residual of the model. The results obtained for DPPH, FRAP and TPC were analysed using this model. The normal distribution of residues and the homoscedasticity of the variances were tested using the Shapiro test and Levene test (car package). The differences between the treatments were tested using the posthoc multiple comparative HSD test (agricolae package) with an alpha of 0.05.
Physical characterisation of the powders depending on drying method
In terms of all the physical characteristics, no differences were observed between the different tested lees matrices. Therefore, only the results obtained for one sample of lees are presented here (Lees 1). The all results of physical characterisation of lees powders are shown in supplementary data (Figures S1-S7). Moisture Content (MC) and yield were evaluated and compared for the different drying techniques, except for VO, because it was difficult to collect all the hardened dry matter as it had stuck to the trays; therefore, the values would have been underestimated and we preferred not to take them into account.
First, the performance of the drying methods in terms of MC was evaluated. The MC value is important, because a lower residual MC could contribute to better conservation and preservation of antioxidant activity during the longterm storage of dried powders. The FD samples had the lowest MC (from 1.34 ± 0.2 to 2.0 ± 0.1%), with some differences depending on the operating conditions. In fact, performing primary drying at higher shelf temperature (FD2: primary drying at 20°C) resulted in higher MC (FD2: 2%) than FD1 (1.3%) and FD3 (1.6%) (primary drying at -30°C). Using FD process, most of the water is removed via frozen water sublimation during primary drying. Vapour pressure at the surface of frozen water is directly proportional to drying temperature and thus a higher secondary drying temperature during FD will result in lower MC [31]. The difference in terms of secondary drying temperature between FD1, FD3 (25°C) and FD2 (20°C) may explain the higher drying performance of FD1 (1.3%) and FD3 (1.6%). This MC values fall within the classical range obtained for FD [19].
For SD, MC was higher than that obtained for FD and was temperature dependent: a decrease in MC from 5.2% to 3.0% with an increasing inlet temperature of 120°C to 200°C respectively was observed (Table 1). These values are in agreement with those reported in other studies on SD [17,32,33]. These authors explained that using SD under high temperature conditions increases the heat transfer rate and consequently particles with reduced MC are formed.
Next, the process yields of FD and SD were calculated. As expected, very high yields were obtained using FD (about 99%), being significantly higher than with SD (about 28-59%). No differences were observed between the different FD cycles, but yield significantly increased with increasing inlet temperature using the SD process (Table 1). These yield values are in agreement with the results reported in other studies [1,17,34]. The low yields obtained with SD can be explained by the adhesion of particles to the drying chamber walls, making them difficult to collect at the end of drying. In fact, it has already been shown that the nature and the molecular weight of dried compounds may be linked to the amount of such particle adhesion [17].
In a second step of this study, the impact of the drying process on the powder particle sizes was assessed. First of all, all the lees were observed using SEM after coarse redispersion. All the SD powders, regardless of inlet temperature, were found to have the same morphology, with spherical entities (around 6 μm in diameter) corresponding to the yeasts, which are the major component of wine lees (Figures 1 and 2). Slightly larger aggregates (around 10 μm in diameter) covered by yeasts to varying extents were also observed on the micrographs. Unlike the SD powders, the FD powders showed agglomerated matter corresponding to yeasts, along with plates approximately 100 μm and 1 μm thick, which probably corresponded to the tartaric acid present in the sample (Figure 3). The results of the analysis of particle size distribution by laser diffraction are consistent with the SEM observations. For the SD samples, the particle sizes ranged from 1 to 10 μm, with a mean value of 6 μm. This powder morphology and mean particle diameter is consistent with that found in classical SD powders [35,36]. Regarding FD samples, a large proportion of the particles corresponded to the aggregates observed in the SEM, with a mean particle size of around 100 μm (Table 2).
Figure 2. SEM images of Freeze Dried (FD) and Vacuum Oven dried (VO) lees 1: A) FD1, B) FD2, C) FD3 and D) VO. For FD1, freezing was carried out at -50°C and primary drying at -30°C; for FD2, freezing was carried out at -50°C and primary drying at 20°C; for FD3, freezing was carried out at -80°C and primary drying at -30°C.
Figure 3. Particle size distribution (after coarse redispersion for FD samples or grinding for VO sample) determined using laser diffraction particle size technique. Results are expressed as Volume density (%) as a function of size (μm). Spray Drying (SD) was carried out with an inlet temperature of 120°C, 160°C and 200°C. Freeze Drying (FD) was carried out as follows: FD1: freezing at -50°C and primary drying at -30°C; FD2: freezing at -50°C and primary drying at 20°C; FD3: freezing at -80°C and primary drying at -30°C. Vacuum Oven (VO): 50°C and 1 Pa.
| SD120 | SD160 | SD200 | FD1 | FD2 | FD3 | VO | |
|---|---|---|---|---|---|---|---|
| Yield (%) | 28 | 37 | 59 | 99.9 | 99.5 | 98.2 | N.D. |
| Moisture content (%) | 5.2 ± 0.3 | 4.1 ± 0.3 | 3.2 ± 0.2 | 1.3± 0.2 | 2.0 ± 0.1 | 1.6 ± 0.2 | 3.5 ± 0.3 |
Considering the powders obtained using the Vacuum Oven (VO) and after grinding, the SEM results showed lots of larger aggregates than in the two other cases. These aggregates looked quite dense and were of around 200 μm in size. Yeasts were also visible on their surfaces, but dispersed differently than in the preceding cases. These morphological and size differences do not have an impact on the solubility of the powdered lees. Their antioxidant properties were then determined, as described in the following section.
Antioxidants properties
It is well known that drying temperature can have a significant effect on antioxidant compounds [17,37]. The antioxidant capacity of four different fresh (i.e., before being dried) lees matrices was first evaluated using DPPH (antiradical power) and FRAP (Ferric Reducing Capacity) methods. Then, the impact of the different drying processes on antioxidant power was assessed and compared with the antioxidant capacity of fresh lees (Figure 4). Only the results from one of the lees matrices are shown (Lees 1) since similar results were obtained from all the four studied samples. The all results are shown in Supplementary Data (Figures S7 and S8). The antioxidant activity of dried lees was evaluated using both DPPH and FRAP assays, since any single assay may give a reduced and suggestive assessment of antioxidant power and needs to be interpreted with caution. In addition, the chemical complexity of a lees matrix [38] can lead to contradictory results depending on the assay carried out [39]. These methods are based on different mechanisms that measure overall antioxidant power: the DPPH assay is based on the ability of antioxidants to act as radical scavengers and the FRAP assay measures the potential of antioxidants to perform as reducing agents by ferric (oxidation catalyst) reducing power.
Figure 4. Antioxidant capacity of lees before and after the different drying processes, according to results of A) DPPH and B) FRAP assays, expressed as mg TE.g DM-1. FL=Fresh Lees before drying; FD1=Freeze Drying with freezing at -50°C and primary drying at -30°C; FD2=Freeze Drying with freezing at -50°C and primary drying at 20°C; FD3=Freeze Drying with freezing at -80°C and primary drying at -30°C; SD120= Spray drying at 120°C; SD160= Spray drying at 160°C; SD200= Spray Drying at 200°C; VO = Vacuum Oven at 50°C.
Figures 4A and 4B shows the impact of Freeze-Drying (FD), Spray-Drying (SD) and Vacuum Oven (VO) on the antioxidant capacity of lees (Lees 1) compared to fresh lees.
The antiradical activity (Figure 4A) of fresh lees was 9.5 ± 0.5 mg TE.g DM- 1. The values obtained for SD lees dried at 120°C, 160°C were significantly higher (by about 15%) than those obtained for fresh lees. This increase may be due to the SD lees being more dispersed in the DPPH reactive than the fresh lees. When comparing SD lees dried at 120°C, 160°C and 200°C it can be seen that the process temperature impacted the DPPH values for SD lees. At 120°C and 160°C, the values for SD were similar and no significant difference was observed: 11.6 ± 0.8 and 11.2 ± 1 mg TE.g DM-1 respectively. At a temperature of 200°C, SD showed a 23% decrease in DPPH value (SD200: 8.8 ± 1 mg TE.g DM-1) compared to the lower (120°C and 160°C) temperatures. This decrease may be due to the thermal alteration of the compounds involved in antiradical power. The DPPH values of FD1, FD2 and FD3 were between 9.6 ± 0.4 and 10.6 ± 0.8 mg TE.g DM-1. No significant differences were observed between FD samples and the fresh lees. However, a statistical difference was observed between the fresh lees and the lees dried via the VO process (DPPH: 11.8 ± 0.5 mg TE.g DM-1).
However, the FRAP assay (Figure 4B) gave different results depending on the drying method. The fresh lees showed a FRAP value of 34.2 ± 2.2 mg TE.g DM-1. This value is comparable (no statistical difference) to VO and FD2. A drop of about 25% was observed for FD1 and FD3, as well as for SD120 and SD160. A higher loss (44%) in FRAP power was observed for SD200. Regarding the FD process, the results indicate that performing primary drying at a higher temperature (20°C) than in the other cycles (-30°C) is beneficial for the preservation of antioxidant properties. This effect may be due to the shorter drying time of primary drying at higher temperatures.
Thus, the FRAP and DPPH assay results exhibited the same tendencies and they confirm that only the SD process at a high temperature (>160°C) seriously affects the antioxidant activity of dried lees. Bioactive compounds extracted from winemaking by-products, including polyphenol compounds, have been found to be heat-sensitive and degradable at temperatures of around 190°C [40]. It is therefore possible that the antioxidant compounds in the wine lees were degraded as a result of exposure to high temperatures during the SD drying process. This could explain why SD at a low temperature, FD and VO helped to maintain the bioactive compounds in the white wine lees. Decreases in the antioxidant power of bio-compounds following thermal treatments has already been reported in other matrices, like those of plant samples, vegetables and fruits [16,17,41]. This reduction in antioxidant activity has been attributed to the thermal degradation of biomolecules and thus of their bioactivity [37]. The values related to antioxidant potential (DPPH and FRAP) obtained in this study are comparable to those of antioxidant compounds obtained from other natural sources [16,19,42] this indicates that white wine lees are a promising new source of antioxidant compounds.
In order to evaluate the stability of the antioxidant activity of dried lees, DPPH assays were also carried out 12 months after drying (Figure 5 and Figure S9). The statistical analysis shows that, in general, dried lees maintained the same DPPH values after 12 months of storage in the dark at room temperature. Only SD120 and FD2 showed significant differences after storage: a 14% increase and a 6% decrease respectively (p<0.05). The results of this stability evaluation show that drying is essential for preserving and maintaining the antioxidant capacity of lees over time. Fresh lees naturally evolve over time due to the enzymatic phenomenon of yeast autolysis (autolysis kinetics is modulated by storage temperature) which can modulate its composition and therefore its initial antioxidant capacity [38].
Figure 5. Antioxidant capacity (DPPH) of lees 12 months after drying. Legend: FD1= Freeze Drying with freezing at -50°C and primary drying at -30°C; FD2= Freeze Drying with freezing at -50°C and primary drying at 20°C; FD3= Freeze Drying with freezing at -80°C and primary drying at -30°C; SD120= Spray Drying at 120°C; SD160= Spray Drying at 160°C; SD200= Spray Drying at 200°C; VO=Vacuum Oven at 50°C; *: Statistically significant (alpha of 0.05).
Since the antioxidant capacity of lees is often related to the polyphenol compounds they contain [1], Total Polyphenol Content (TPC) was determined using the Folin-Ciocalteu method (Figure 6 and Figure S10). This method is based on the transfer of electrons in alkaline solution from phenolic compounds to phosphomolybdic/phosphotungstic acid complexes. The results show that TPC for all dried lees by different methods varied between 46.4 ± 1.3 mg GAE.g DM-1 and 39.7 ± 0.3 mg GAE.g DM-1. These values fall within the same range as those found for other winemaking products, such as wine grape pomace and skins [23], or for other matrices, such as herbs used as phytochemicals [16,42]. A higher concentration of polyphenol compounds (46.4 ± 1.3 mg GAE.g DM-1) was found in SD lees dried at 120 and 160°C. The TPC values for all FD conditions were slightly lower (41.9 ± 0.3 mg GAE.g DM-1) than the one obtained with SD lees dried at 120 and 160°C (from 46.4 mg GAE.g DM-1 to 39.7 mg GAE.g DM-1). The lowest TPC value was observed for SD at 200°C (39.7 ± 0.3 mg GAE.g DM-1) and for VO at 50°C. These results are partially in line with those obtained for antioxidant capacity of lees dried by SD at 200°C using DPPH and FRAP. In the light of this, the evolution of polyphenol concentrations could be linked to the antioxidant potential of lees, but it is also possible that other compounds are involved in antioxidant power, as described by Romanet R, et al. [43]. Processing methods are known to have varying effects on total polyphenol compounds and on the antioxidant properties of molecules [44,45] including the decrease in or enhancement of antioxidant activity. Drying processes that involve increasing temperatures can either modulate the properties of natural antioxidants present in the matrix or induce the formation of new compounds with antioxidant power, so that overall TPC, DPPH or FRAP values either increase or remain unchanged [45,46].
Figure 6. Total Polyphenols Content (TPC) of dried lees. Legend: FD1= Freeze Drying with freezing at -50°C and primary drying at -30°C; FD2= Freeze Drying with freezing at -50°C and primary drying at 20°C; FD3= Freeze Drying with freezing at -80°C and primary drying at -30°C; SD120= Spray Drying at 120°C; SD160= Spray Drying at 160°C; SD200= Spray Drying at 200°C; VO= Vacuum Oven at 50°C.
The analysis of the results of the DPPH, FRAP and TPC analyses show that the most appropriate drying methods were SD at inlet temperature in the range of 120-160°C and FD, which, out of the different tested methods, resulted in the best preservation of antioxidant capacity and total polyphenol content. From a performance point of view, FD leads to a higher yield (almost 100%) and lower moisture content (<2%) than SD at inlet temperature lower than 160°C (yield<50% and MC around 4-5%). VO resulted in the preservation of antioxidant compounds, despite the lower TPC, but its implementation in our experiments was neither easy nor repeatable and supplementary experiments would be needed to improve the performance of the VO process.
From an economical point of view, FD with higher freezing temperatures (-50°C instead of -80°C) and with higher primary drying temperatures (20°C instead of -30°C for the first cycle) is of interest as it requires less expensive drying conditions for its implementation. In the same way, using SD at low temperatures could be the first choice for the production of antioxidant capacity products from biomass and in continuous mode, with lower costs involved than the FD process [47].
Drying is a useful technique for extending the shelf-life of compounds of interest, such as antioxidants from agro- and bio-sourced products. This study evaluates the impact of different drying processes (SD, FD and VO) and their operating conditions on the physico-chemical properties and antioxidant activity of white wine lees. The results showed that each of the three methods of drying only slightly affects the physical properties of the obtained powders. FD generally resulted in larger particles due to aggregation, without any impact on the dissolution of the powders prior to analysis of their antioxidant capacity. In addition, the FD, VO and SD (<160°C) drying processes had no impact on the antioxidant capacity of the fresh lees, making it possible to preserve stability for 12 months at room temperature. The antioxidant capacity of the lees was found to be partly related to their polyphenol concentrations after assessment using the Folin Ciocalteu method. In order to better understand the compounds involved in the antioxidant capacity of white wine lees, additional studies on other families of compounds must be conducted. Finally, of all the tested drying processes, the highest yield (>98% of dry matter) was reached using FD. It can be concluded that white wine lees could be a new potential source of antioxidants for food, nutraceuticals, pharmaceuticals or cosmetics products.
The authors would like to acknowledge “Fonds de dotations des oenologues de France”, “SansoVin project” funded by Region Nouvelle- Aquitaine and Biolaffort and the National Research Agency (ANR Valoli) for providing financial support. We also thank Warren Albertin for helping with the statistical analysis.
Benjamin Poulain: Methodology; writing of original Draft; Writing - Review and Editing; visualisation.
Hassana Hsein: Experimental design, methodology, writing of original draft, writing-review and editing, supervision.
Pierre Tchreloff: Experimental design, methodology, writing of original draft, writing-review and editing, supervision, project administration.
Claudia Nioi: Experimental design, methodology, writing-original draft, writing-review and editing, supervision, project administration, funding acquisition.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Journal of Bioprocessing & Biotechniques received 3351 citations as per Google Scholar report