Review Article - (2022) Volume 12, Issue 6
Received: 15-Feb-2022, Manuscript No. JCDE-22-55576;
Editor assigned: 17-Feb-2022, Pre QC No. P-55576;
Reviewed: 03-Mar-2022, QC No. Q-55576;
Revised: 18-Apr-2022, Manuscript No. P-55576;
Published:
27-Apr-2022
, DOI: 10.37421/2165-784X.22.12.455
Citation: Dahal, Sanjaya. "Synthesis of Alkali Activated Geopolymer Cement from Clay." J Civ Environ Eng 12 (2022) : 455.
Copyright: © 2022 Dahal S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The term "geopolymer" was created and applied by Davidovits, a French scientist, in 1979 to represent a kind of inorganic polymer with SiO4 and A1O4 tetrahedral being the structural units. In general, geopolymers as a class of inorganic polymer are formed by reaction between an alkaline solution [e.g., sodium hydroxide and sodium silicate] and an aluminosilicate sources such as metakaolin, fly ash, and slag. Nowadays, geopolymer studies are receiving commendably increasing attention because they may be used as a viable economical alternative to organic polymers and inorganic cements in diverse applications, such as military, aircraft high-tech ceramics thermal insulating foams fire-proof building materials protective coatings refractory adhesives and hybrid inorganicorganic composites. This interest is due to their exceptionally high thermal and chemical stability, excellent mechanical strength, adhesive behavior and long-term durability. In addition, early researcher have demonstrated that geopolymers are cheap to produce and can be made from a great number of minerals and industrial by-products, including pozzolana, natural aluminosilicate minerals, metakaolin fly ash granulated blast furnace slag fly ash and kaolinite mixture fly ash and metakaolin mixture red mud and metakaolin mixture and red mud and fly ash mixture. Moreover, they are environmentally friendly materials from the point of view of reducing green house effects caused by CO2 emission from the manufacturing of Portland cement.
Geopolymer • Davidovits • Aluminosilicate • Metakaolin
The chemical composition of geopolymer material is similar to natural zeolitic materials, but the microstructure is amorphous instead of crystalline According to geopolymers possess amorphous to semi-crystalline three dimensional silicoatelutrmahineadtrea bsytr ucsthuarerisn g coanlsl isttihneg ooxf ygliennke adt omSs,i O4w hicahn d canA 1Obe4 designated as poly-sialate (-Si-O-Al-O-) (Si:Al=I), poly-sialatesiloxo (-Si-O-Al-O-Si-O-) (Si:Al=2), poly-sialate-disiloxo (-Si-OAlO- Si-O-Si-O-) (Si:Al=3), and sialate links (Si:Al>3). The sialate is an abbreviation for siliconoxo-aluminate. There is also an empirical formula for geopolymer matrix:
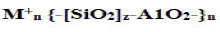 (1)
(1)
Where M+=an alkali cation [K+,Na+] for balancing the negative charge of Al3+ in IV-fold coordination; n=degree of polymerization; and z=Si/Al ratio. By varying the Si/Al ratios (i.e., z=1-15, up to 300) geopolymers exhibit different properties: low ratios [i.e., Si/ Al<3] result in three-dimensional cross-linked rigid networks and stiff and brittle properties (as cements and ceramics); high ratios (i.e., Si/Al>3) results in 2-D networks and linearly linked polymeric structures with adhesive and rubbery properties, respectively. In general, geopolymerization is a complex multiphase process, comprising a series of dissolution reorientation-solidification reactions.
The generation of reactive species or alkali activation, which is the dissolution of amorphous phase’s alurninosilicates by alkali to produce small reactive silica and alumina? Reorientation, which is the transportation or orientation or condensation of precursor ions into oligomers and the actual setting reaction, which is the polycondensation process leading to the formation of amorphous to semi-crystalline aluminosilicate polymers. However, these three steps can overlap with each other and occur almost simultaneously, thus making it difficult to isolate and examine each of them separately.
Cements are composed of Portlandite [Ca(OH)2] and calcium silicate hydrade (C-S-H) phases, while geopolymer cement is based on 3 dimensional alumino silicate frame work. The geopolymer cements has the following advantages in comparison to cement based composite material.
• Resistance against acid
• Temperature resistance
• High strength
• Low shrinkage
• Corrosion resistance
• Fire resistance
• High durability
• Cold setting
• Quick setting
• Simple manufacturing techniques
• Availability of abundant raw materials
• Reduced energy consumption
• Reduced greenhouse gas emission
For the chemical designation of geopolymers based on silicoaluminates, poly (sialate) was suggested. Sialate is an abbreviation for silicon-oxo-aluminate. The sialate network consists of SiO4 and AlO4 tetrahedra linked alternately by sharing all the oxygens. Positive ions (Na+, K+, Li+, Ca++, Ba++, NH4+, H3O+) must be present in the framework cavities to balance the negative chargeof Al3+ in IVfold coordination. Thus, poly (sialates) are chain and ring polymers with Si4+ and Al3+ in IV-fold coordination with oxygen and range from amorphous to semi-crystalline. Some related frame works are shown in Figure 1.
Phosphate-based geopolymer
Phosphate ceramics are synthesized at room temperature and they set rapidly like conventional polymers. They contain naturally occurring mineral phases, notably apatite. They represent another variety of mineral geopolymer where Si is totally or partially replaced by P. They are formed by an acid-base reaction between a metal oxide and an acid phosphate. Virtually any divalent or trivalent oxide that is sparingly soluble may be used to form these phosphate geopolymers. They have found a wide range of applications such as dental cements, construction materials, oil well cements and hazardous and radioactive waste stabilization. The main difference between the silicate based geopolymers and phosphate geopolymers, however, is their syntheses. Poly (sialate) geopolymers and their derivates are synthesized in alkaline environment but phosphate geopolymers are fabricated by acid-base reactions.
Phosphate geopolymers
A very wide range of phosphate geopolymers may be synthesized by acid-base reaction between an inorganic oxide (preferably that of divalent and trivalent metals) and an acid phosphate. The reaction product is generally a poly (hydrophosphate) or an anhydrous poly (phosphate) that consolidates into a ceramic. The following are the most common examples.
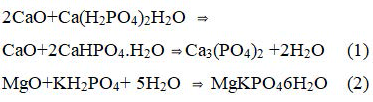
These reactions occur at room temperature. By controlling the rate of reaction. Ceramics can be formed. With trivalent oxides. Similar ceramics can he formed at a slightly elevated temperature. A good example is berlinite (AlPO4), which is formed by the reaction between alumina and phosphoric acid:
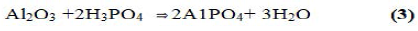
It was also demonstrated that phosphate geopolymers of trivalent oxides such as Fe2O3 and Mn2O3 might be produced by reduction of the oxide and then acid-base reaction of the reduced oxide with phosphoric acid. The reaction may be described by the following equation:
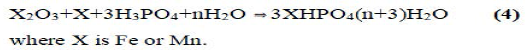
High-molecular phosphate-based geopolymers: cristobalitic AIPO4
Berlinitc (AIPQ4) is the only known mineral to the isostructural with quartz. Isostructural means that they have the same structure although the two minerals have rather different chemistries. Quartz, SbO2, would seem to be very different from berlinite, AlPO4. But if the formula of quartz is written as SiSiO4 instead of 2(SiO2) then the similarity is obvious. The reason that berlinite is able to have the same structure as quartz is because the aluminum and phosphorus ions are of similar size to silicon ions with following bond lengths Si-O 1,63 A, PO 1,63 A, Al-O 1,73 A. Thus the same structure can be achieved since tire aluminums and phosphorus can completely replace the silicons without alteration of the quartz structure. The cristobalite form of aluminum phosphate may be obtained by heating the normal berlinite form of aluminum phosphate at an elevated temperature which is preferably in excess of 1000°C. The synthesis of cristobalitic (highmolecular) AlPO4 geopolymers follows two different routes. Time first process includes sol-gel chemistry whereas his second system involves the reaction between phosphoric acid and metakaolinite MK-750.
Organo-mineral geopolymers
Silicone: The similarity of the siloxane (Si-O-Si) structure in organo-silicones to the chains, rings, and networks of silicon and oxygen found in silica and the silicate minerals, for example in quartz has been pointed out many times. Almennigen et al. reported the correspondence in a study of disiloxane H6Si2O. As observed by Noll it is possible to pass from the polymeric silicate to the polymeric covalent molecules of an organosiloxane by replacing the bridging oxide ions of the silicate anions with meth I groups. The structures that result from this replacement closely resemble the silicate and aluminosilicate molecules: monomers, dimers, trimers, etc, rings, chains. Sheets and frameworks of corner-sharing silicate [SiO4] groups. When the organic radical is methylene the structures of the oligomeric poly-methyl-siloxanes are identical with those of poly (siloxonate) (Si-O-Si-O) and poly (sialate) (Si-O-Al-O-Si) geopolymers.
Hybrid organo-mineral geopolyrners: This new class of compounds was first obtained by incorporating the geopolymer into the organic polymer structure, adapting the chemical composition of the components. For example a hi-functional epoxy resins. Diglycidyl Ether of Bisphenol A (DGEBA). Was mixed with 20 wt% of MK-750 based geopolymer slurry, with a curing agent in an aqueous medium. The resulting hybrid material has excellent mechanical properties and improved fire resistance. The new developments are focusing on improving the mechanical and physical properties of the geopolymer itself, however, both organic and geopolymer phases are physically incompatible. Obtaining a homogeneous mixture without phase separation requires a new approach.
Humic-acid based: kerogen geopolymer: Working on the transformation of geomolecules through geochemical processes during diagenesis, have drawn attention to the concept of geopolymer in association with kerogen and petroleum. Kerogen-geopolymer is the most stable material and the final alternating product in the Earth. Some geopolymeric materials can last for a long time due to their unique geopolymeric structure, so-called three-dimensional crosslink. Geopolymers can be classified into two major groups: pure inorganic geopolyiners and organic containing geopolymers, synthetic analogue of naturally occurring macromolecules. The small content of organics is a key parameter governing the strength and durability of material in a large volume of inorganics. Organic compounds can be incorporated into refractory macromolecules such as lignin and inelanodin or humic materials. Huinic materials represent an inorganic-organic structure (Figure 2).
Diagenesis of organic matter leads from biopolymers synthesized by organisms through “humin” to Kerogen, a geopolymer, by partial destruction and rearrangement of the main organic building blocks (Figure 2). Kerogen is considered to be the major starting material for most oil and gas generation as sediments are subjected to geothermal heating in the subsurface. It is the most. Abundant form of organic carbon on Earth about 1000 times more abundant than coal, which forms primarily from terrigenous remains of higher plants.
Kerogen is a geopolymer that contains a high content of organics. Kerogen geopolymers generally occur in numerous forms: sonic have more organics and less inorganics, while others had the opposite. It is, however, evident that both inorganics and organics are required in a mix at a certain ratio. Which will result in a geopolymeric structure? This geopolymeric structure exhibits a similar organization to human bone and teeth. Typical inorganic-organic composites that show extreme durability and mechanical strength. The mechanism of geomacromolecule formation involves the crosslink reaction between the inorganic and organic materials. Cements are composed of portlandite [Ca(OH)2] and calcium silicate hydrate (C-S-H) phases, while geopolymer cement is based on an 3 dimensional aluminosilicate framework. The comparisons between the Portland cement and geopolymeric cement are given below Figure 3.
This difference is important in 2 respects:
• Alumino-silicate materials are much more resistant to chemical attack than calcium rich Portland cement.
• The calcinations step (heating to 1450°C) is not required in the synthesis of geopolymer cement thus avoiding the release of CO2.
Hence, if it is possible to synthesize geopolymer of reasonable compressive strength from coal fly ash waste, it can be a suitable alternative for minimizing the waste as well as for reducing the atmospheric pollution. The geopolymers have following advantages in comparison to cement-based composite materials are:
Statement of problems
Large amount of cement is required due to the rapid urbanization and population, growth. Due to which excessive amount of OPC has been produced but it may cause production of excessive amount of CO2 which caused the global warming. In addition there is negative impact on the environment such as:
• Consumption of large amount of energy in order to produce cement.
• The emission of large amount of CO2.
• Red clay contains huge amount of Silica which caused deterioration of the soil quality.
• Deterioration of natural resources.
Objectives of the study
Extensive work on geopolymer research has been conducted so far. Previous researchers suggested that more efforts should be made to investigate the geopolymers induced under some particular reaction conditions, such as utilization of different raw materials and low temperature synthesis. Therefore, this study is focused on a developing field that utilizes cheap and plentiful aluminosilicate materials or industrial byproducts to make geopolymers with excellent mechanical, chemical, and physical properties as well as long-term durability at ambient environment. Its main objectives are:
General objectives
Innovation: Induce geopolymers using the waste mixtures that have never been reported in the existing literature.
Reuse of industrial wastes: Use geopolymer technology to convert industrial by-products into useful materials
Potential applications of the synthesized geopolymer: Construction materials and waste containment and stabilization.
Specific objectives
• Investigation of the synthesis process of the geopolymers manufactured by red clay, rice husk ash and wide clay.
• Examination of the composition, microstructure, and mechanical properties of the resulting geopolymeric products.
• Exploration or validation of the potential applications of the end geopolymeric materials in terms of their water absorption, bulk density, apparent porosity.
Basics of geopolymer technology
The French scientist, Joseph Davidotis, invented and first used the term, geopolymer, on the basis of consisting of Al and Si which are both essential geological structural elements Geopolymers should be considered as a new material, new binder, or new cement for concrete Although different terminology (e.g., low-temperature aluminosilicate glass, alkali-activated cement, and hydro ceramic) have been used by researchers, "geopolymer. It has been more than 30 years since Davidotis published the earliest paper on a geopolymer study in 1979. On the other hand, the geopolymer science has been studied for more than 3 decades so far, but it was investigated in very few laboratories and institutions in the first 20 years. During the period of 1979 to 1999, there are merely about 91 journal papers and patents as well as one conference proceedings (Geopolymer 99) that were published on the subjects of geopolymers and geopolymerization. However, there was a significantly booming world-wide increase in geopolymer research in the most recent 10 years. For instance, one country, China, produced approximately 135 scientific papers dealing with geopolymer science and technology in a single year, 2010. This has been linked with geopoymers' wide variety of potential applications, which include fire resistant materials, decorative stone artifacts, thermal insulation, low-tech building materials, low energy ceramic tiles, refractory items, thermal shock refractory, bio-technologies materials for medicinal applications, foundry industry, cements and concretes, composites for infrastructures repair and strengthening, high-tech resin systems, radioactive and toxic waste containment, arts and decoration, cultural heritage, archaeology and history of sciences [1].
To date, the properties and uses of geopolymers are being explored in many scientific and industrial disciplines as well: modern inorganic chemistry, physical chemistry, colloid chemistry, mineralogy, geology, and in all types of engineering process technologies. The first innovation on geopolymerization was done on kaolinite and metakaolinite by Davidovits, according to him, alkaline liquid used to react with silicon and aluminium in a source material of geological origin or in by product materials such as fly ash and rice Huskash to produce binder. This process is polymerization which formed at low temperature and short time by naturally occurringalumino-silicates. Many works have been done in geopolymer field by researcher by using different raw materials such as coal fly ash, rice husk ash, bottom ash, red mud, etc.
Red mud the industrial waste produced by the Bayer's process for the extraction of alumina from bauxite ores, one of the oldest largescale industries in the world. According to US Geological Survey report, bauxite are mined globally amounts to 202 Million Tons (MT). Red Mud characterized by strong alkalinity even with higher water content up to 95% consisting of an excessive amount of dissolved sodium Hydroxide used to extract silicates and alumina. Red mud mainly includes iron oxides and some toxic heavy metals. Two major environmental concerns for the safe and economical disposal of red mud is due to strong alkalinity and higher water content. Different research work have been conducted for the utilization of Red mud. But a widely accepted technology is yet to be developed. Due to high ph, heavy metals, radio activity, the RM might cause environmental problems so, a new technologies utilizing Red mud as a raw materials for manufacturing high added value products are urgently needed. A new type of geopolymer composite was synthesized from Red Mud (RM) and Rice Husk Ash (RHA), at a varying mixing ratios of raw materials.
The influence of synthesis factors including chemical composition of raw materials and curing conditions, on the microstructure and mechanical properties of geopolymers synthesized from Red Mud (RM) and class F Fly Ash based (FFA) was investigated. New geopolymer formulations were designed by sodium silicate/NaOH activation of metakaolin, iron oxide and red mud mixtures. The effect of source materials on the microstructure and mechanical properties were studied. Each formulation induces degree of geopolymerization reaction as reflected by the phase composition whereas the amorphous phase is predominant. The effect of source materials on the microstructure and mechanical properties were studied by comparing two types of geopolymers synthesized from metakaolin, a non-waste materials, and the admixture of two waste, red mud and fly ash. Red mud, a residue of Bayer’s process was used synergistically with fly ash to develop geo-polymer. An influence of 0-40% red mud addition on the reaction, structure and properties of fly ash geopolymer was studied. An improvement in setting time and compressive strength was observed. One part “just add water” geopolymer binders are synthesized through the alkali-thermal activation of the red mud which is relatively rich in both alumina and calcium. Calcination of the red mud with sodium hydroxide pellets at 8000 C leads to decomposition of the original silicate and aluminosilicate phases present in red mud, which promotes the formation of new compound with hydraulic character.
The potential use of red mud for the synthesis of inorganic polymeric materials through geo-polymerization was studied. The geo-polymerization process involves a chemical reaction between red mud and alkali metal silicate solution under highly alkaline solution leads to the formation of a compound having high compressive strength, very low water absorption, and excellent fire resistance. One-part geo-polymer was synthesized from alkali-thermal activated Bayer Red Mud (RM) with addition of silica to optimize its composition. Red mud based geo-polymers were formed and their potential was investigated to ensure prolonged pH control. Several properties of the novel geo-polymers were examined including buffering ability, alkali leaching behaviour, mineralogical composition, micro structure and physical properties. A composite of geo- polymeric material was synthesized from Bayer red mud combined with granulated blast furnace slag. Thermal pre-treatment was applied to improve the solubility of red mud in alkaline solution to promote geo-polymerization. A new type of geo-polymer composite was synthesized from two industrial wastes; Red Mud (RM) and Rice Husk Ash (RHA) at varying mixing ratios of raw materials. Prolonged curing significantly increases the compressive strength and Youngs modulus, but reduces the ductility. Higher RHA/RM ratios generally lead to higher strength. The strength and microstructure of two geopolymer synthesized from metakaolin, a non-waste material and the admixture of two wastes, red mud and fly ash. Unconfined compression test was conducted to access their curing time. The production and characterization of bauxite and red mud in view of world. It reviews comprehensively the disposal and neutralization methods of red mud gives the detailed assessment of the work for the utilization of red mud in geo-polymer, clay material, and cement ceramic.
Well reacted geo-polymers with good compressive strength (44-58 Mpa) were formed highly alkaline residue from red mud without the addition of strength promoting components such as fly ash by adjusting the composition to an optimal SiO2/Al2O3 ratio of about 3 with silica fumes. The comprehensive utilization of red mud and gives the detailed assessment of work carried until now for the utilization of red mud in different fields. X-ray Diffraction (XRD), Fourier Transform Infrared (FTIR) spectroscopy, Scanning Electron Microscopy (SCM) and compressive strength measurements were performed to fully characterize metakaolin-based geo-polymers with Si/Al ratios 1.12, 1.77 and 2.20 in order to establish structure performance relationship in this system. The durability and heavy metal leaching behaviour of red mud-class F Fly ash based geo-polymers (RFFG) were investigated. RFFG specimens were soaked in sulphuric acid solution (pH=3.0) and de-ionized water (pH=7.0) for 1-120 days. The different types of industrial wastes such as biometric ash, red mud, recycled glass and heavy metals waste in their application for the geo-polymer production [2].
Mechanism of geopolymerization
Geopolymerization is an exothermic process that is carried out through oligomers [dimer, trimer] which are the fundamental unit structures for the three dimensional macromolecular edifice Davidovits also stated that geopolymerization could be regarded as the analogue of synthesis of zeolite. In other words, the chemistry involved in geopolymerization is close to that in synthesis of zeolite, although the geopolymer microstructure is amorphous to semicrystalline rather than crystalline. In general, geopolymerization involves a number of processes including dissolution, reorientation, and solidification [3].
During the dissolution step, both Si and Al. species are produced when Si-Al raw materials come in contact with alkaline solution. stated that the extent of generation of both Si and Al were contingent upon the following aspects: concentration of the alkaline solution, alkali metal cation (e.g., Na, K) in alkaline solution, mixing rate and time, and intrinsic properties (e.g., structure and composition) of Si-Al raw materials. It is believed that, of all these stated factors, the concentration of alkaline solutions and the intrinsic properties of the Si-Al raw materials are dominant. Throughout the reorientation step, the dissolved Si and Al species (e.g., Al3, Si") are diffused into the oligomers. Plus, the oligomers in the aqueous phase form relatively large networks by condensation, resulting in the formation of a gel. Meanwhile, the further leaching of reactive Al and Si species from the raw materials is occurring when the dissolved Al' and S14 + on the surface of source Si-Al materials are removed. According to the time and intensity of stirring are main factors for this step. Longer leaching period and a more intense stirring can maximally remove the dissolved Si and Al species from the surface of raw materials and kinetically break the barrier between the Si-Al particle surface and the gel phase so as to accelerate the reorientation of both Al and Si species. At the step of solidification, the gelation system continues to rearrange and reorganize, as the connectivity of the gel network increases, resulting in the amorphous or semi-crystalline three-dimensional aluminosilicate network commonly attributed to geopolymer. At this stage, temperature and air circulation are two major factors determining the properties of the final geopolymeric products It needs to be pointed out that there is no specific order for these 3 major steps. In other words, they occur simultaneously. For instance, during the step of solidification, both dissolution and reorientation are happening as well. Glukhovsky during 1950s proposed a general mechanism for alkali activation of materials comprise of silica and reactive alumina. The Glukhovsky model divides the process into three stages.
• Destruction-coagulation,
• Coagulation-condensation and
• Condensation-crystallisation
More recently, researchers have elaborated and extended
Glukhovsky theories and applied the accumulated knowledge about zeolite synthesis to explain the geopolymerisation process. The reaction mechanism shown in the Figure 3 outline the key processes occuring in the transformation of a solid aluminosilicate source into a synthetic alkali aluminosilicate (Figure 4).
Dissolution of the solid aluminosilicate source by alkaline hydrolysis produces aluminate and silicate species (most likely in the monomeric form) into solution has always been assumed to be the mechanism responsible for conversion of the solid particles during geopolymerisation. The species released by dissolution are incorporated into the aqueous phase, which may already contain silicate present in the activating solution. A complex mixture of silicate, aluminate and aluminosilicate species is thereby formed. It was proposed that the chemical mechanism takes place in each step of geopolymerisation process and can be summarized into the following 5 steps.
Dissolution reaction: Dissolution involves the formation of mobile precursors through the complex action of hydroxide ions. Aluminosilicates are dissolved in the alkaline solution to produce Si and Al monomers.
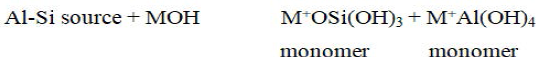
Diffusion: Al and Si species diffuse into the gel phase after leached from the surface of the aluminosilicate particle. This reduces the concentration of Al and Si species at the particle surface which enhance further dissolution from the surface.
Polymerization: Polymerization of monomers then take place to form the highly ordered dimer which further reacts with another monomer or dimer or Si-oligomers to form higher oligomers of varying geometries, i.e linear, branched or cyclic.
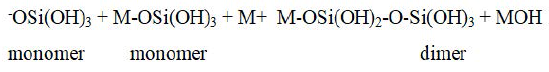
The polymerization between Al complex and Si complex will take place in preference to the polymerization between the Si complex since the activation energy for forming an Al-O-Si linkage is lower than that of Si-O-Si linkage.
Formation of aluminosilicate gel: Formation of (-Al-O-Si-) bond network by reacting the M+ and the higher Si-oligomer.
Polycondensation and hardening process: It was believed that the geopolymeric gel was transformed to the final structure either through another dissolution and crystallization or solid-state mechanism. However, at this stage of hardening there is no major movement of particles, but leaching and diffusion between particle surface and the gel phase may still occur and a slight movement of paste in capillary pores may also take place. All reaction steps take place simultaneously and it is difficult to isolate each reaction step for detailed examination. The chemical mechanism can be interpreted in the following way:
Step 1: Alkalination and formation of tetravalent Al in the side group sialate -Si-O-Al-(OH)3-Na+,
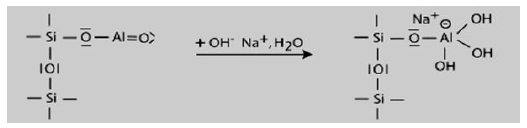
The alkalination takes place in the first step of mechanism which results in the tetravalent aluminum group formation.
Step 2: Alkaline dissolution starts with the attachment of the base OH to the silicon atom, which is thus able to extend its valence sphere to the penta-covalent state.
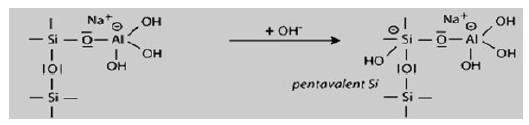
The hydroxide then attacks the attached silicon in the second step, taking it to a pentacovalent state and a negatively charged central Si atom. This leads to the cleavage of the second Si as a silanol (Si-OH) group.
Step 3: The subsequent course of the reaction can be explained by the cleavage of the siloxane oxygen in Si-O-Si through transfer of the electron from Si to O, formation of intermediate silanol Si-OH on the one hand, and the basic siloxo Si-O- on the otherhand.
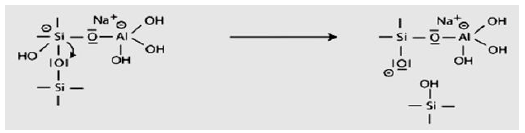
The third step of the mechanism leads eventually to the production of ortho-sialate molecule.
Step 4: Further formation of silanol Si-OH groups and isolation of the ortho-sialate molecule, the primary unit in geopolymerisation
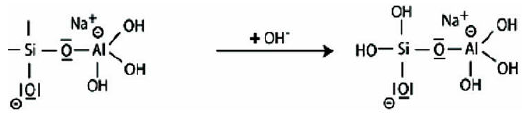
Step 5: Reaction of the basic siloxo Si-O- with the sodium cation Na+ and formation of Si-O-Na terminal bond.
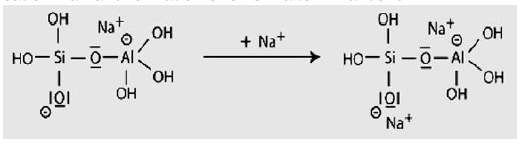
Step 6a: Condensation between ortho-sialate molecules, reactive groups Si-ONa and aluminum hydroxyl OH-Al, with production of NaOH, creation of cyclo-tri-sialate structure, whereby the alkali NaOH is liberated and reacts again and further polycondensation into Napoly (sialate) nepheline framework.
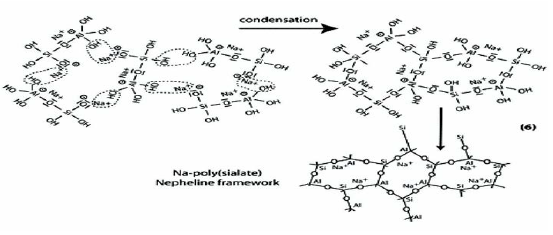
Step 6b: In the presence of water-glass (Soluble Na- Polysiloxonate) one gets condensation between di-siloxonate Q1 and ortho-sialate molecules, reactive groups Si-ONa, Si-OH and aluminum hydroxyl OH-Al-, creation of ortho-sialate-disiloxo cyclic structure, whereby the alkali sodium hydroxide is liberated and reacts again.
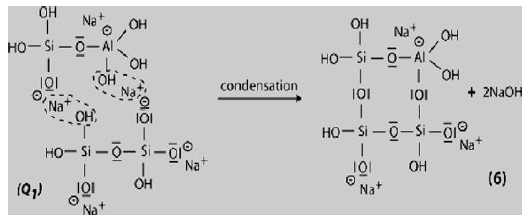
In this step, the condensation results in a complex framework with a cyclo-tri-sialate and ortho-sialate-disiloxo structure that doesnot easily line up in perfectly crystalline rows.
Step 7: Further polycondensation into Na-poly (sialate-disiloxo) albite framework with its typical feldspar crankshaft chain structure.
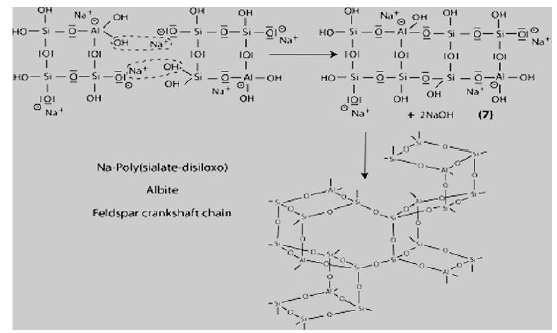
Successful use of geopolymer concrete in different applications such as building products, reinforced concrete beams, fire resistance materials, railway sleepers, encapsulation of toxic metals, high temperature materials, etc., has been reported by many researchers. Some challenges that are faced by this new technology are lack of long term durability data, lack of standard methods which measure the performance of geopolymers, and conservative nature of construction industry. Since 1986, the French aeronautic company Dassault Aviation has been using geopolymer mold and tooling in the development of the Rafale fighter plane. In Australia, on September 28, 2014, the newly completed Brisbane West Well camp airport becomes the greenest airport in the world. More than 30,000 cubic meters, cement-free geopolymer concrete was used to save more than 6,600 tons of carbon emissions in the construction of the airport. The University of Queensland’s Global Change Institute (GCI) is the world’s first building to successfully use geopolymer concrete for structural purposes [4].
Raw materials used to make geopolymers
White clay (Kaolinite): Kaolinite is a 1:1 clay mineral (Figure 1) with the chemical composition Al2Si2O5[OH]4, which means each particle has one tetrahedral silica layer and one octahedral alumina layer. Individual particles of kaolinite form stacks with hydrogen bonds and van der Waals forces holding together successive particles. The strength of these bonds prevents water from entering the interlayer spaces and causing swelling. Cation Exchange Capacity [CEC] values for kaolinite typically ranges between 3 to 15 meq/100 g. Kaolinite also has a low shrink-swell capacity. It is a soft, earthy, usually white mineral, produced by the chemical weathering of aluminum silicate minerals like feldspar. Rocks that are rich in kaolinite are known as china clay, white clay, or kaolin. Kaolin is a fine, white, clay mineral that has been traditionally used in the manufacture of porcelain. It is thought that the term kaolin is derived from the Chinese Kaoling (Figure 5).
Metakaolin is a dehydroxylated form of the clay mineral kaolinite associated with the reaction Al2Si2O5[OH]4→Al2O2SiO2 + 2H2O. Between 100-200°C, kaolinites lose most of their adsorbed water. In the range of 500-800°C, kaolinites become calcined by losing water through dehydroxilization. The dehydroxilization of kaolinite to metakaolin is an endothermic process due to the large amount of energy required to remove the chemically bonded hydroxyl ions, which breaks down the crystal structure producing a transition phase (silica and amorphous alumina in reactive form) with high surface area. Metakaolin is a highly pozzolanic and reactive material. Burning at higher temperature will cause recrystallization into quartz and mullite investigated the effect of heat treatment parameters on the dehydroxylation process of the kaolinite-based materials such as natural and artificial kaolin clays with different amounts of amorphous phase [metakaolin]. At calcination temperature below 450°C, kaolin showed relatively low level of the dehydroxylation degree, less than 0.18. In the range from 450 to 570°C, the degree of dehydroxylation sharply increased to 0.95, and finally between 570 and 700°C, the kaolinite was fully dehydroxylated. The dehydroxylation was accompanied by kaolinite amorphization, which affected the activity of additives. The development of pozzolanic properties in fired clays mainly depends on the nature and abundance of clay minerals in the raw material, the calcination conditions and the fineness of the final product. The calcination temperature producing the reactive state is usually in the range of 600˚C-800˚C. On heating, recrystallization and formation of metakaolin (2SiO2∙Al2O3) or mullite (3Al2O3∙2SiO2) takes place, resulting in a decline of material reactivity.
It was reported that Metakaolin (MK) is highly reactive metastable clay that is essentially an anhydrous aluminosilicate obtained from calcining kaolin at around 650˚C-700˚C. The reactivity of metakaolin varies with thermal treatment, during calcinations (450˚C-600˚C) and turns into metakaolin, a material with some degree of order. In metakaolin, the Si-O network remains largely intact while the structure of Al-O network is reorganized. After all, metakaolin offers good properties as supplementary cementing material. The geopolymerisation of metakaolinite activated by alkali and alkali silicate solutions was extensively studied. From the study it was revealed that the geopolymerisation process of metakaolinite under alkali activation can be occurred in three stages: (I) destruction, (II) polymerization and (III) stabilization shown in the Figure. The influence of mechanical activation of raw kaoline on the final compressive strength of as obtained geopolymer. Mechanical activation was performed by dry ball-milling of raw kaolin at 250 rpm for 1 h. Mechanical activation was performed to improved mechanical properties. Result showed that without mechanical activation, optimum curing condition was 24 h at 70°C and compressive strength was 15 Mpa after 28 days [5].
Red mud: Red mud is the major industrial waste produced by the Beyer process for the extraction of alumina from bauxite ores, one of the oldest large-scale industries in the world. Karl Joseph Bayer, an Austrian chemist, applied for a patent on the digestion of bauxite, an aluminium-containing ore, by means of a concentrated sodium hydroxide solution employed at elevated temperature and pressure, which enables dissolution of the aluminium content and its separation from other bauxite components. Depending on the quality and purity of the bauxite ore, the quality and purity of the bauxite ore, the quantity of red mud generated varied from 55-65% of the processed bauxite. According to a recent US Geological Survey of the processed report (2009), bauxite ore mined globally amounts to 202 million tons. As such, there are approximately 120 MT of red mud produced in 2008 [6].
Red mud is characterized by strong alkalinity even with high water content [up to 950] owing to the presence of an excessive amount of dissolved sodium hydroxide used to extract silicates and alumina. Although red mud varies in physical, chemical and mineralogical properties due to differing mineral sources and refining processes adopted, rust hue is an intrinsic property of all red mud, which is caused by the oxidized iron present in the mud. In addition, solid constituents of red mud include mainly iron oxidized heavy metals. It also contained radioactive minerals. Strong alkalinity and high water content a safe and economical disposal of red mud. Thus, its treatment and disposal are a major difficulty to alumina refineries. Although intense research work on utilization of red mud was conducted during previous decades, a widely accepted technology that can be employed to recycle red mud is not available at present. In the past, red mud was disposed from the plant site mainly two ways, including dumping it directly into the sea or onto the land creating huge ponds. Due to the intrinsic properties [e.g., high pH, heavy metals, radioactivity], the above disposal methods caused significantly environmental problems to the surrounding communities. Therefore, new technologies utilizing red mud as a raw material for manufacturing high added-value products are urgently needed. There was a catastrophic red mud disaster occurred in Hungary on 10/04/2010. A so-called tailing dam that held waste products, including arsenic and mercury, from the Ajkai Timfoldgyar aluminnum-processing plant in the town of Ajka, Hungary, collapsed. This released an estimated 184 million gallons (697 million liters) of highly alkaline red mud into the Marcal River and nearby towns, killing at least eight people and seriously harming hundreds of residents and the surrounded environment. Hungary Prime Minister Viktor Orban called the spill the country's biggest ecological disaster and stated there would not be vegetation in the contaminated area for quite a long time. Thus, new safe and environmentally-friendly disposal methods for red mud are urgently needed which is one of major purpose of this work. The primary work on geopolymer was done on kaolinite and meta-kaolinite by Davidovits, according to him alkaline liquid used to reacts with silicon and aluminium in a source material of geological origin or in by-product materials such as fly ash, red mud to produce binder. This process is polymerization, which formed at low temperature and short time by natural occurring alumino-silicates. Red mud based geopolymers and their potential to ensure prolonged pH control were evaluated. The lower pH gradient over 28th day (1.64 pH units) was achieved by red mud. A high and prolonged buffer capacity was accomplished, proving that RM-based geopolymers had potential to be applied as pH buffering material. Geopolymer from Red Mud. In this research, strength of the specimens increases for replacement of 5%, 10%, 15% RM and strength of specimens starts decreases and hence, optimum percentage of cement replaced by Red Mud was determine to be 15% whose 7 days compressive strength was 14.12 MPa, 28 days compressive strength was 38.23 MPa [7].
Rice Husk Ash (RHA): Rice husk, also called rice hull, is the hard protecting covering of grains of rice, which is a by-product generally obtained from milling process of rice crop. The RHA is generated after burning the rice husk in the boiler, which is collected from the particulate collection equipment attached upstream to the stack of rice-fired boilers. According to a RHA market study (2003), Rice covers 1% of the earth's surface and is a primary source of food for billions of people. Globally, approximately 600 million tons of rice is produced each year. Thus, tons of rice husk are generated. On average 20% of the rice paddy harvest is husk, giving an annual total production of 120 million tons. For the transition from rice husk to RHA, the quantity of RHA generated is about 20% of the processed rice husk. The RHA is highly porous and lightweight with a very high external surface area and contains silica in high content [usually 90-95 wt.%]. At present, the most common method of disposal of RHA is dumping on waste land, thus creating an environmental hazard through pollution and land dereliction problems. Since the amount of RHA generated is in plenty annually, an effective way of disposal of RHA is needed urgently [8].
Raw materials
The raw materials used for the geopolymer synthesis include Red Clay (RC) which was obtained from Kavrepalanchok district, Banepa-11, Rice Husk Ash (RHA) from rice mill factory from Banepa-5 and White Clay (WC) from Suryabinayak.
Activator solution
Sodium hydroxide [97% Qualigens chemicals, India] and liquid Na2SiO3 [Na2O-7.6%, SiO2-26.0%] were used as alkali-activator solution.
Sample preparation
Red clay, white clay and Rice Husk ash were first dried, mixed and then activator solution was added. Different samples were prepared shown in the following Table 1, Figure 6.
| Sample No | RC | RHA | WC |
|---|---|---|---|
| M1 | 1 | 0 | 0 |
| M2 | 0.95 | 0.05 | 0 |
| M3 | 0.9 | 0.1 | 0 |
| M4 | 0.85 | 0.15 | 0 |
| M5 | 0.8 | 0.2 | 0 |
| M6 | 0.7 | 0.3 | 0 |
| M7 | 0.6 | 0.4 | 0 |
| RW1 | 0.95 | 0 | 0.05 |
| RW2 | 0.9 | 0.05 | 0.05 |
| RW3 | 0.9 | 0 | 0.1 |
| RW4 | 0.8 | 0.1 | 0.1 |
| RW5 | 0.8 | 0 | 0.2 |
| RW6 | 0.7 | 0 | 0.3 |
| RW7 | 0.6 | 0 | 0.4 |
The samples were cured for 60°C for 3 hours and in closed oven for 24 hours. The samples were then removed from the moulds and kept for different time duration at lab temperature [20-25°C]. Finally, the compressive strength of thus obtained geopolymer products was measured on CBR machine (Figures 7 and 8).
Characterization techniques
X-Ray Diffraction [XRD] analysis: The X-Ray Diffraction (XRD) pattern of the raw material, geopolymeric sample were done. The sample was powered and was scanned from 10 to 80° [20] for sample. The XRD patterns were recorded on X-Ray Diffractometer [D2 phase Diffractometer, Bruker Germany] using CuKα radiation available at Nepal Academy of Science and Technology [NAST] Khumaltar, Lalitpur.
Fourier Transform Infrared [FTIR] analysis: Fourier Transform Infrared analysis of powdered raw material, geopolymoric sample was performed using FTIR spectrophotometry (IR tracer 100, Shimadzu, Japan) in the frequency range of 4000 to 400 cm-1 at Custom Department, Tripureshwor, and Kathmandu (Figure 9).
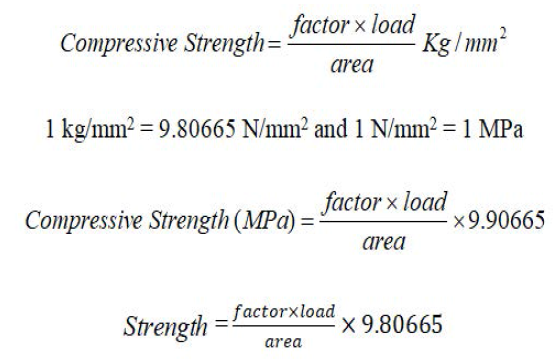
Compressive strength of the geopolymer products: The compressive strength of the prepared geopolymer, sand and geopolymer mixture cube was measured by using CBR machine as shown in fig at Central Material testing Laboratory, IOE, Pulchowk Campus, TU. Each data of the compressive strength presented in this study is the average of three consecutive measurement Compressive strength is the capacity of a material or structure to with stand loads tending to reduce size. The compressive strength is the unioxial force applied in the unit square meter. It is the form of applied pressure. It is an most important property for all concrete and a major indicator of general quality control. Factors influencing the strength of concrete include the types and quality of materials, the mixture proportion, the construction method, curing condition and test method. It also depends upon curing time and curing temperature (Figure 10). When the curing timed temperature increases, the compressive strength increase. It can be calculated by using following formula:
Physical properties of the geopolymer products: Water absorption, apparent porosity, Bulk density were determined as per ASTM 373, the geopolymer was first dried in oven at 150°C for 2 hours and was allowed to allowed to cool and dry weight was taken. The geopolymer was then boiled for 5 hours in water bath and allowed to soak for additional 24 hours and weight was measured.
XRD analysis
The X-ray diffraction pattern of RC, RHA, WC, and RC based geopolymer.
The XRD patterns of the sample were plotted between 2θ angles 20 to 80°. The peaks around 2θ=21, 27, 5, 5, 6, 69° represent the presence of quartz (Silica). The entire picks were prominent in case of geopolymer mixture. Some major picks at 2θ=25.4, 28.7, 31.8, 35.7, 36.6, 37.5, 460 were observed was mainly due to calcium silicate, calcium silicate hydrate, calcium aluminosilicates. The raw materials as well as geopolymer product obtained from it were almost amorphous in nature except the quartz peak appearing at 2θ=27.50. The quartz peak was broadening in case of its geopolymer product. The hump appearing in the range of 2θ=22-350 also conversion of crystalline to amorphous nature of the raw materials to geopolymeric product. The diminishing XRD peaks of the raw materials after the treatment with NaOH and sodium silicate is due to the dissolution of aluminosilicate and the formation of geopolymer products, while same crystalline (Silica) transform into semicrystalline phases and remained as silica after geopolymerization process.
Chemical composition of raw materials
The chemical composition of Red clay, Rice Husk ash and white clay (Table 2, Figure 11).
| Elements | Si | Fe | Al | K | Ca | Ti | S | Mn |
|---|---|---|---|---|---|---|---|---|
| RC [%] | 41.788 | 28.547 | 15.02 | 9.428 | 2.881 | 0.775 | 0.608 | 0.46 |
| RHA [%] | 56.07 | 18.524 | 13.44 | 5.643 | 2.708 | 1.649 | 0.694 | 0.33 |
| WC [%] | 57.368 | 21.457 | 8.088 | 7.192 | 2.357 | 2.234 | 0.671 | 0.12 |
FTIR spectrum
The FTIR spectrum of RC, RC based geopolymer in the spectrum of RC based geopolymer showed main absorption bands at 675, 990, 1000, 1390, 2350, 3597 and 3724 cm-1 . The bands in between 990, 1000 cm-1 are the major fingerprints of the geopolymeric mixture and attributed to the Si-O-Si and Al-O-Siasymetric vibration which shows the formation of amorphous aluminosilcate gel phase due to the dissolution of raw material under highly alkaline condition. The peak at 1390 cm-1 due to the stretching vibration of O-C-O bond indication the atmospheric carbonation. The bands around 450-780 Cm-1 detected are due to in plane Si-O bending and Al-O linkage as well as bending Si-O-Si and O-Si-O vibration. The band at 3597 cm-1 represents the stretching and bending [H-O-H] vibration (Figure 12).
The FTIR spectrum of red clay based sample displays a sharp band at 3640 cm-1 associated to O-H stretching vibration of calcium hydroxide, a weak band at 960 cm-1 correspond to S-O4 stretching mood and out-of-plane bending mood of ettringite respectively. A solder near 530 cm-1 is due to out-of-plane Si- O bending vibrations. The geopolymer product shows larger absorption band near 1450 cm-1 and of the small one at 870 cm-1 correspond to antisymmetric stretching and out-of-plane bending moods of CO3 2- ions respectively. The observation suggest that geopolymers are less sensitive to atmospheric carbonation. The principal band associated with Si- O(Al) stretching vibration in SiO4 tetrahedral near 1000 cm-1 is very broad for geopolymer which suggest geopolymers are more disorder. It also appears that this Si-O stretching band shifts progressively towards greater wave number for geopolymer sample (Figure 13).
Optical microscopy image
The Figure 13 shows the micromorphological features of the three raw materials. The red clay is characterized by irregularly shaped aggregates. The compact structure shows the formation of geopolymer binder.
Compressive strength
The compressive strength of geopolymer samples were done with different parameter variation for 28 days and are shown below
Variation of NaOH concentration: The bar graph shown below shows the change in compressive strength with the variation of sodium hydroxide (Figure 14).
The plot shows the influence of NaOH concentration on the RC and RHA geopolymer clearly, shows different mechanical properties resulted from varied NaOH concentrations 4, 6 and 8 M can be identified. It is commonly agreed that alkalinity is one of the most significant factors affecting the properties of geopolymers since they are alkali activated materials. In this study, however it appear that alkali concentration may cause variable mechanical behavior which differs from previous observations that higher viscosity of higher NaOH concentration hinders the leaching of silicon and aluminium, resulting in a negative effect on the degree of polymerization and hence the mechanical properties of final product the excess OHconcentration from higher NaOH concentration solution cause aluminosilicate polymerization is hindered resulting in the influence on the mechanical properties and as the RHA's in completed dissolution and reaction is believed to cause significant variability in the mechanical properties of final product. So, NaOH concentration is the critical factor affecting in mechanical properties of geopolymer [9].
Variation of curing time at different RC and RHA percentage
The variation of RC and RHA mixed ratio with NaOH was done with the addition of sodium silicate as activator solution. The raw material mixed ratios were M1, M3, M5, M6, M7 as shown in the sample designation. The addition of sodium silicate has greatly enhanced the mechanical strength of the geopolymer specimen (Figure 15).
Above, graphs shows that compressive strength has been increased in 7 days with addition of sodium silicate and has reached to about 18 MPa. Such trend in compressive strength variation at the sample gives the indication that the improvement in strength is related to the amount of Na2SiO3 used. Compressive strength gets significantly increased in 7 days and gets increased upto 28 days. The significant increment in the compressive strength was due to red clay. The above results show that red clay only can be used as the construction material.
Variation of curing time at different RC and WC percentage
At 28 days of curing the compressive strength of the sample RW3, RW5, RW6 and RW7 were found to 10.12, 11.16, 5.05 and 6.1 respectively. The above plot shows that the Geopolymer samples RW5 and RW3 shows higher compressive strength, this result may be due to the increase in the reactivity due to the addition of white clay (Figures 16 and 17).
Variation of curing time at different RC, WC and RHA percentage
The above plot shows the compressive strength of Geopolymer sample varied between RC, WC and RHA with different mixed ratio [RW1 and RW2]. The most important and significant mixture of raw material was found to be the mixture of RC, Wc and RHA ratio. The compressive strength was obtained upto 34 MPa in 28 days. The compressive strength has increased from 7 days and upto 28 days it has been in increasing rate. The increment in strength was due to higher silica containing property of white clay which binds the material to obtain high value.
Physical property: The durability of the building unit is greatly influenced by water absorption. Lower the value of water absorption higher is the resistivity to the possibility of water infiltration and environmental damage. Water absorption, apparent porosity and bulk density were carried of 28 days as per ASTM 373 standard is mention in the Table 3. From these result, the water absorption values were found less than 17%, a limit of ASTM. 90 standard specifications for load bearing masonry unit. This shows that the composition of the products is suitable for the preparation of the building units (Table 2) [10].
| Sample No | Bulk Density | Water Absorption |
|---|---|---|
| M1 | 2.1 | 7.04 |
| M2 | 2.01 | 8 |
| M3 | 2 | 8.04 |
| RW1 | 1.76 | 8.38 |
| RW2 | 2.6 | 5.88 |
| RW3 | 2.03 | 8.18 |
The FTIR and XRD analysis supported the formation of geopolymeric and cementitious materials and indicated microstructural change between them. The compressive strength of RedClay with sodium hydroxide variation (4 M, 6 M, 8 M) gives maximum strength at 4 M at 7 days. The raw material variation for red clay and rice husk ash gives the maximum strength was obtained to 24 MPA for 28 days. The raw materials variation for red clay and white clay gives maximum strength of 11 MPA. For RW5 sample. The raw material variation for red clay, white clay and rice husk ash gives maximum strength for the sample RW2 upto 34 MPA for 28 days. There are many challenges that are faced by this technology. Those are lack of long term durability data, lack of standard method which measure the performance of geopolymer and conservative nature of construction industries. If it is possible to prepare geopolymer of reasonable compressive strength with red clay, it can become a suitable alternative to replace OPC.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Journal of Civil and Environmental Engineering received 1798 citations as per Google Scholar report