Research - (2020) Volume 7, Issue 2
Received: 19-Mar-2020
Published:
19-Apr-2020
, DOI: 10.37421/2380-2391.2020.7.265
Citation: Itodo AU, Sha Ato R, Wuana RA, and Emmanuel A Yerima. "Effect of Phytoremediation on PAHs Levels of Agricultural Soil around Mechanic Village Wukari, Nigeria". J Environ Anal Chem 7 (2020) doi: 10.37421/jreac.2020.7.265
Copyright: © 2020 Itodo AU, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
This study evaluates the PAHs composition in agricultural soils around Mechanic village, Wukari using standard procedures by means of GC-MS. The possible source of the PAHs in the soil was deduced, using the diagnostic ratio analysis for PAH origin. Risk assessment was based on the incremental life cancer risk of PAHs proposed by Provisional Guidance for Quantitative Risk Assessment of the United States Environmental Protection Agency. Effect of phyto-remediation using Zea mays inter-planted with Striga hermonthica (SMV-MS), Zea mays alone (SMV-M), Zea mays inter-planted with Striga hermonthica alongside the application of fertilizer (SMV-MSF) and Zea mays alone alongside fertilizer application (SMV-MF). The result reveals that the PAHs composition based on ring prevalence in agricultural soils around Mechanic village Wukari was in the order Σ5 >Σ6 >Σ4 >Σ3 >Σ2 ring. Dibenz [a,h] anthracene 4.52 μg/kg (39.09%) has the highest percentage abundance but was less than the 100 μg/kg and 700 μg/kg Canadian soil quality guideline for agricultural and commercial layout while acenaphthene 0.111 μg/kg (0.90%) was the least abundant. The source of the PAHs in the soil was basically pyrogenic based on the diagnostic ratio analysis while phytoremediation of the soil using Zea mays inter-planted with Striga hermonthica significantly reduce the PAHs content of the soil.
PAHs • Composition • Source • Toxicity • Phytoremediation
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic pollutants which enters the environment through natural processes such as forest fires, volcanic activity and anthropogenic means via incomplete combustion of fossil fuels and accidental leakages of petroleum products such as coal tars [1]. PAHs have been reported to have carcinogenic activity and they have been included in the European Union (EU) and the Environmental Protection Agency (EPA) lists of priority pollutant. A lot of studies has carried on Benzo (a) pyrene (BaP) due to its carcinogenicity and is now use as a reference to ascertain the carcinogenic potency of other polycyclic aromatic Hydrocarbons (PAHs) [2]. Transport and distribution of PAHs in the environment are mainly governed by their physicochemical properties. Low molecular weight PAHs (2 to 3 fused aromatic rings) are more soluble in water than those with higher molecular weight PAHs (more than 3 fused aromatic rings) and are usually distributed in soil and groundwater more readily than the later. These low molecular weight PAHs may occur in the atmosphere mainly as vapor due to greater values of Henry’s law constant. They are prone to degradation process in the environment. High molecular weight PAHs, due to less water soluble and high lipophilic characters are usually adsorbed to particles in the environments [3]. Soil is the primary steady reservoir and sinks for PAHs in the terrestrial environment, because PAHs are readily absorbed by organic matter in soil where they remain relatively stable [4]. The accumulated PAHs in soil organic matter provides potential for PAHs to find its way into food chains where toxic PAHs may exert their toxic effect on human health [5-7].
In view of the ubiquitous nature and toxicity of PAHs, various techniques have been employed to reduce and control PAHs and other organic pollutants in the environment such as chemical degradation [8], photo degradation, which has been reportedly enhanced using nano-composites catalyst [9,10]. As well as biodegradation for instance rhizospheric biodegradation which is based on the secretion by plants root exudates which supports the growth and metabolic activities of diverse fungal and bacterial communities in the rhizosphere capable of degrading varied pollutants. The secreted enzymes can transform the chemicals around the rhizosphere without the need for uptake of the pollutants for detoxification. Rhizospheric microorganisms have been reported to decontaminate areas by volatilizing pollutants or by increasing the production of humic substances from the organic pollutants [11,12].
This study intends to evaluate the PAHs composition in agricultural soils around Mechanic village Wukari, the possible source of the PAHs in the soil using the diagnostic ratio analysis, the incremental life cancer risk of PAHs and the effect of phytoremediation using Zea mays inter-planted with Striga hermonthica.
Study area and sample collection
Mechanic village Wukari with the geographical coordinate 7°51’ 17.208” N and 9°47’ 40.374” E is situated in Wukari local government area of Taraba state, Nigeria. The facility has been in existence for about two decades now where activities such as car repairs, car spraying, car electrical components fittings, car battery repairs and others are carried out on daily basis there by generating a lot of waste where in most cases such waste are done away with by means of incineration.
Stratified sampling technique was used for soil sample collection where the sampling site was broken into four (4) stratums (small areas) north, south, east and west with respect to Mechanic village Wukari. Each stratum was further subdivided into four quadrants of equal size before five (5) samples were taken randomly by grab method within the depth of 0-15 cm in the individual quadrant (smaller area) making a total of twenty (20) samples per strata (small area) and a total of eighty (80) samples from the four stratums situated at the north, south, east and west of the industry to enable detailed representation of variability within the study area. The 80 sample units of approximately equal size were pooled together to form the composite and a representative sample for the entire area labeled SMV [13,14].
The representative soil sample obtained was sorted to eliminate pebbles and coarse materials and then air-dried at room temperature over three days with occasional breaking of aggregated materials with wooden roller; followed by sieving through a nonmetallic sieve with mesh hole of 2 mm diameter to remove stones, plants and animal’s debris. The pH and soil textural class were determined by standard methods described by the United State Department of Agriculture [15,16] while the soil organic carbon content was determined by titrimetry after wet oxidation of soil sample using potassium dichromate and concentrated sulphuric acid. Since the average content of carbon in soil organic matter is equal to 58% the conversion factor 1.724 was used to calculate the percentage of organic matter from the content of organic carbon [17,18] (Figure 1).
Phytoremediation experiment
The composite soil sample collected from the farmlands around mechanic village Wukari labelled SMV before remediation was further divided into four portions of about 4.0 L each in a pot and were placed in the greenhouse. To about 4.0 L of the first portion of soil in a pot, 5 seeds of maize were sown to about 2-3 cm depth of soil without striga and about 1.884 g of NPK fertilizer was applied as amendment after 3 weeks of planting to aid the development of maize plant. After harvest the maize, the soil was labeled SMV-MF, to the second portion of the soil in a pot, 5 seeds of maize were sown to a depth of about 2-3 cm without amendment after 3 weeks of planting to serve as control. After harvesting the maize, the soil was labeled SMV-M. To the third portion of the soil in a pot, 50 g of the striga seed in 50 g of the soil were homogenized to help serve as carrier since the striga seed is extremely tiny before sprinkling them onto the soil followed by sowing of 5 seeds of maize to about 2-3 cm depth of the soil in the pot. About 1.884 g of NPK fertilizer was applied as amendment after 3 weeks of planting to aid the development of maize plant as describe by Berner in striga research methods manual [19]. After harvesting plant tissues even though striga could not germinate, the soil was labeled SMV-MSF. Likewise, to the last soil portion in a pot, 50 g of the striga seed in 50 g of the soil were homogenized before sprinkling them onto the soil followed by sowing of 5 seeds of maize to about 2-3 cm depth of soil in the pot, without amendment after 3 weeks of planting to serve as control. After harvesting the maize plant tissues as striga could germinate, the soil was labeled: SMV-MS.
PAHs analysis
Chemicals: All solvents and reagents used were of trace analysis (TA) and chromatographic grade. Standards of 16 PAHs were obtained from Sigma Aldrich Chemical Company. Internal and surrogate standards were used for sample quantification. PAHs working standards, internal standard mixture solutions and surrogate standard mixture solutions were properly diluted with GC grade n-hexane and prepared daily before the analysis. Glassware was washed before use with n-hexane and dried in an oven at 105°C.
Sample preparation for polycyclic aromatic hydrocarbon analysis
Exactly 20 g of the homogenized, sieved and pre dried soil samples was heated at 40-60°C for 4 hours and cool to remove any trace of moisture before extraction. The soil samples were extracted for 30 minutes using solvent by means of ultra-sonication where 2.0 g of the dried soil sample was weighed and transferred into a 50 mL glass conical flask containing 10 mL mixture of acetone and dichloromethane 1:1 (v/v) then capped and placed in an ultrasonic bath (Grant XUBA3) in which four samples could be extracted simultaneously. The extraction step was repeated twice, and the resulting extracts were combined and filtered through a Whatman filter paper No. 41. The combined extract was concentrated to near dryness using rotary evaporator, transferred into amber vial and further concentrated by means of nitrogen concentrator (LabTech E.T. Parallel) [20,21].
The soil extracts containing the PAHs were purified by column chromatography packed with silica gel and anhydrous sodium sulphate after saturation with 2.0 mL of acetone and dichloromethane 1:1 (v/v). Each extract was loaded onto the column and eluted with dichloromethane. The first 1.0 mL of eluate was discarded before 5.0 mL of eluate was collected into an amber coloured vial and the PAHs content analyzed by means of GCMS [21,22].
Quality assurance and quality control for PAH analysis
The following PAH compounds (internal standard mixture) was used to pre-spike the sample extracts: Napthalene, 2-methyl Naphthalene, Biphenylene, Acenapthene, Anthracene, Phenanthrene, Triphenylene, Fluorene, Fluoranthene, Pyrene, Benzo(a)anthracene, Benzo(b)fluoranthene, Benzo(a)pyrene, Dibenzo(a,h)anthracene, Indeno(1,2,3-cd) pyrene and Benzo(ghi)perylene. The base peak ion was use as the primary ion for quantification of the standard compounds. Where interferences are noted, the next two most intense ions were used as the secondary ions. The internal standard compound was added to all calibration standard solutions and all sample extracts to be analyzed by gas chromatograph.
Three concentration levels calibration standard solutions of PAHs were prepared by adding appropriate volumes of one or more stock standard solutions to a volumetric flask. The stock standard solution was prepared from certified solution of 2000 μg/mL of each analyte of interest. The stock standard solution was prepared at concentrations of 100 μg/mL in toluene and 100 μg/mL of the internal standard added.
The calibration table was constructed from instrument responses for target compounds at concentrations of 25, 50 and 100 μg/mL. To each calibration standard solution, the calibration standard solutions was at a concentration near but not above the maximum detection limit and the other concentrations were ensured to correspond to the expected range of concentrations found in real samples or define by the working range of the GC system. The working calibration curve was checked each working day by the measurement of one or more calibration standard solutions. Where the response for any analyte varies from the predicted response by more than ± 10%, the test was repeated using afresh calibration standard solution [23].
Limits of detection (LOD) were determined as signals 3 times the background signal. Peaks that were smaller than 3 times the signal-to-noise ratio were not considered. The LOD for PAHs ranged from 10 to 500 pg g-1. The average recoveries of PAHs were 80-110% for 10 soil samples.
GC-MS conditioning
An Agilent Technologies GC-MS comprises of 7890A gas chromatography and MS 5975C mass spectrometer detector was used in this study. The instrument comprises a HP 5 MS column of length 30 m, thickness 0.25 μm, internal diameter of 0.32 mm and helium as carrier gas at the rate of 1 mL/min. Oven temperature programme of initial temperature at 60°C hold for 1 minute then ramp to 240°C at the rate of 10°C/min to final temperature at 300°C hold for 6 minutes.
Risk assessment of PAHs in soil
The incremental lifetime cancer risk (ILCR) was employed to evaluate the potential risk of PAHs in agricultural soils around Mechanic village Wukari and Metal works Wukari. The ILCRs for adults in terms of direct ingestion, dermal contact, and inhalation were calculated using the following equations [16,24].
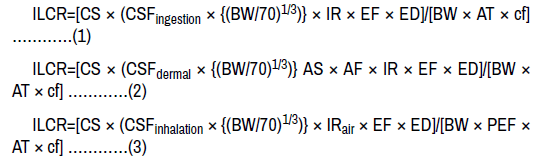
Where CS is the PAH concentration of soils (μg kg-1), which was obtained by converting concentrations of PAHs according to toxic equivalents of BaP using the toxic equivalency factor (TEF). The carcinogenic slope factor (mg kg-1 day-1)-1 (CSF) was based on the cancer-causing ability of BaP: CSFingestion, CSFdermal and CSFinhalation of BaP were 7.3, 25 and 3.85 (mg kg-1 day-1)-1 respectively [25]. BW is body weight (kg): 70 kg; AT is average life span (year): 70 years; EF is exposure frequency (days year-1): 350 days year-1; ED is the exposure duration (year): 30 years; IRsoil is the soil intake rate (kg day-1): 0.0001 kg day-1; IRair is the inhalation rate (m3 day-1): 20 m3 day-1; SA is the dermal surface exposure (cm2 day-1): 5000 cm2 day-1; cf is the conversion factor: 106; AF is the dermal adherence factor (kg cm-2): 0.00001 kg cm-2; ABS is the dermal adsorption fraction (unitless): 0.1; and PEF is the soil dust produce factor (m3 kg-1): 1.32 × 109 m3 kg-1 [7,24,26]. The total risks were the sum of risks of ILCRs in terms of direct ingestion, dermal contact and inhalation.
This study reveals that the PAHs composition in agricultural soils around Mechanic village Wukari was below the Canadian soil quality guideline for agricultural and commercial layout as well as the incremental life cancer risk. The source of the PAHs in the soil was basically pyrogenic based on the diagnostic ratio analysis while phytoremediation of the soil using Zea mays inter-planted with Striga hermonthica significantly reduce the PAHs content of the soil.
Authors have declared that no competing interests exist.
Journal of Environmental Analytical Chemistry received 1781 citations as per Google Scholar report