Research Article - (2022) Volume 6, Issue 2
Received: 22-Feb-2022, Manuscript No. AHBS-22-55148;
Editor assigned: 23-Feb-2022, Pre QC No. P-55148;
Reviewed: 11-Mar-2022, QC No. Q-55148;
Revised: 16-Mar-2022, Manuscript No. R-55148;
Published:
23-Mar-2022
, DOI: 10.37421/ahbs.2022.6.155
Citation: Wodaje, Baye and Samrawit Melkamu. "Study on Prevalence of Lungworm Infection and Associated Risk Factors of Cattle and Sheep Slaughtered at Gondar Elfora Abattoir, North West Ethiopia." J Anim Health Behav 6 (2022): 155.
Copyright: © 2022 Wodaje B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Lungworms are parasitic nematode round worms that infest the lungs of ruminants. The aims of this study were to determine the current prevalence, identify the lungworm species, and assess the possible risk factors of lung worm infection in cattle and sheep at Gondar Elfora abattoir. The study populations were cattle and sheep body condition score, age category, and no sex difference at the Gondar Elfora abattoir, which is male animal used for slaughter. In this abattoir, on average, 85 cattle and 165 sheep are slaughtered per day. The sampling method applied in this study was random sampling techniques from the sampling frame. The total of 500 sampled animals (240 cattle and 260 sheep), both coprological and postmortem examinations were conducted.
Results: Coprological examination; overall occurrence of lungworm infection in cattle and sheep was 3.75% and 7.31%, respectively. The prevalence of infection in young and adult age groups of cattle was 7.04% and 2.37%, and in sheep it was 6.8% and 7.9%, respectively. With regard to body condition (poor, medium and good) having the prevalence of 13.64%, 3.3% and 0.00% in cattle and 16.2%, 5.35% and 8.33% in sheep respectively. Body condition score in cattle has a statistical significant difference (p<0.05). Dictyocaulus viviparous the only prevalent lungworm in cattle (3.75%). In sheep, Dictyocaulus filaria (52.63%) was higher than Muellerius capillaries (36.84%) followed by Protostrongylus rufescens (10.53%). Post-mortem examination; Over all prevalence in cattle and sheep was 1.67% and 8.08%, respectively. The finding with respect to young and adult age groups of cattle was 4.23%, 0.59%, and in sheep it was 7.48% and 8.85% respectively. Age group in cattle has statistical significant difference (p<0.05). The identified lung worm species in sheep, mixed infection (42.85%) was higher than Dictyocaulus filarial (28.57%) followed by Muellerius capillaries (19.05%) and Protostrongylus rufescens (9.52%). Generally, lungworm infection is prevalent in the study site; so it needs emphasis on prevention and control to overcome this problem.
Abattoir • Cattle • Gondar • Lungworm • Prevalence • Sheep
Background
Ethiopia possesses the largest livestock population, being the first in Africa and tenth in the world, with an estimated population of approximately 57.8 million cattle, 28 million sheep, 28.6 million goats, 1.23 million camels, and 60.5 million poultry [1].
In Ethiopia, sheep are the dominant livestock, providing up to 33% of cash income and 23% of the food substance value obtained from livestock production. Sheep play a vital role as sources of meat, milk and wool for smallholder keepers in different farming systems and agro-ecological zones of the country [2]. They are also sources of foreign currency. Sheep and goats account for a quarter of domestic meat consumption, roughly half of domestic wool demand, 40% of fresh skins, and 92% of the value of semi-processed skin and hide exports to other countries. It is estimated that 1,078,000 sheep are used in Ethiopia for domestic consumption annually. There is also a growing export market for sheep meat in the Middle Eastern Gulf States and some African countries. At optimum off take rates, Ethiopia can export 700,000 sheep’s meat annually and at the same time supply 1,078,000 sheep for the domestic market [2]. Ethiopia did not expect any product from these animals due to the prevalence of various diseases that occurred.
Lungworm parasites that infect domestic ruminants are roundworms (nematodes) that belong to the phylum Nemathelminths and are grouped under the Metastrongyloidea and Trichostrongyloidea super families. Of these round worms, Dictyocaulus and Protostrongylus are causes of lungworm infection in ruminants [3]. The epidemiological distribution of lung worm depends more on pasture contamination by carrier animals. In many cases, signs of lungworm infection can range from moderate coughing with slightly increased respiratory rates to severe, persistent coughing and respiratory distress and even failure. Reduced weight gains, reduced milk yields, and weight loss accompany many infections in cattle and sheep. Patent subclinical infections can occur in all species [4].
Lung worm species, particularly in the central and highland areas of Ethiopia, have been shown to be a major problem in small ruminants and cause disease, increased mortality, and production losses [5]. Up to half of all sheep deaths and morbidity on farms in the Ethiopian highlands are caused by parasitic pneumonia and endoparasites [6]. In Ethiopia, there is a single report of lungworm prevalence in cattle from Gondar town by Anne Z. and Gray C, et al. [7]. Lungworms in particular can suppress the immunity of the respiratory tract and cause death, poor weight gain or loss of body weight, as well as greatly affect the potential productivity of sheep in the areas where they are prevalent [8].
To get the expected benefit mentioned above from these animals, prevention and control of lung worm is very important. As per our present state of knowledge of parasitic diseases, it is difficult and even dangerous to put rigid rules for their control work for all regions because the prevalence of respiratory helminthiasis varies from place to place depending on ecological and management factors. For this reason, a study of the epidemiology of each parasitic disease (lung worm infection) should be limited to specific areas of the study area where the study animal's origin is conducive to the lungworm parasite, and lungworm infection is considered an important disease in the study region because of its significant economic loss by decreasing productivity of animals. Hence, The aims of this study were to determine the current prevalence of lung worms in cattle and sheep at Gondar Elfora abattoir, those animals coming from the highlands; to identify the species of lung worm in cattle and sheep that were found in the study area; and to assess the possible risk factors of lung worm infection in cattle and sheep in the study area.
Population under investigation
The study populations in this study were cattle and sheep body condition score, age category, and no sex difference at the Gondar Elfora abattoir, which is a male animal used for slaughter. In this abattoir, on average, 85 cattle and 165 sheep are slaughtered per day. The researchers visited the abattoir two days a week and ten samples were taken per a day during study period.
Study design
Cross-sectional study was conducted from November 2019 to April 2020 to estimate the prevalence, specious identification, and risk factors of lung worm infection in cattle and sheep at Gondar Elfora abattoir, Amhara regional state, North West Ethiopia. Cross sectional survey involving 500 samples (240 cattle and 260 sheep) were examined. The explanatory variables considered were age, specious of animals, and body conduction. Each individual of the sampled sheep and cattle were determined for the presence of lung worm at the time of examination and relevant data collected through clinical and post mortem examination. The body condition of studied sheep was determined by using the 5 point scale (1=very thin to 5=very fat), based on the criteria set by Hendrix CM [9]. In this study the body condition score were categorized in to poor, medium and good. The score from 0 to 1 categorized as poor, 2 to 3 categorized as medium whereas the body condition score from 4 to 5 grouped as good body condition. Whereas Body condition score determination for cattle were Poor (Emaciated, Very thin, Thin), Medium (Borderline, Moderate), Good (Good, Very good, Fat, Overweight), based on the criteria set by Thomson EF and Orita G, et al. [10].
Sampling method and determination of sampling size
The sampling method was random sampling from sampling frame of animals coming to Gondar Elfora abattoir to determine applied in this study the prevalence of lungworm infection and associated risk factors of cattle and sheep. Since the prevalence of lung worm infection of cattle and sheep at Gondar Elfora abattoir was limited study in the earlier (before). So to calculate the total sample size, the following parameters were used: 95% Level of Confidence (LC), 5% desired level of precision, 3.1% [11] prevalence of lung worm in cattle at Gondar district and 20.74% [12] prevalence of lung worm in sheep in different restaurant of Gondar were used in the expected prevalence. The sample sizes were using the formula given in [13].
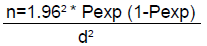
Where,
n=required sample size
Pexp=Expected prevalence
d =desired absolute precision
NB: The authors used an additional number of study animals for this study to enhance the accuracy of the results.
Study methodology
Ante mortem examination: All the slaughtered animals were male and almost local breed. For cattle the age grouping was based on dentition, for those which have not erupted permanent incisor teeth, are classified as young, and while those with pair or more permanent incisor teeth erupted were classified as adult. Body condition score determination for cattle were poor, medium and good, based on the criteria set by Thomson EF and Orita G, et al. [10]. For sheep the age grouping was based on dentition, those which have not erupted permanent incisor teeth, were classified as young, while those with one pair or more permanent incisor teeth were classified as adults [14] and whereas body condition scoring of each animal was determined based on the criteria set by Hendrix [9].
Coprological examination: Total of 500 samples (240 cattle and 260 sheep) were taken from Gondar Elfora abattoir. Faecal samples were collected directly from the rectum of all selected animals using disposable gloves and stored in vials containing 10% formalin and transported to the Gondar parasitology laboratory for examination. During sample collection, date of sampling, the species of the animal, and age were recorded. The collected fecal samples were processed the techniques recommended by Mulate B and Mamo M [15] were employed for identification of lung worm species from the collected samples. In the laboratory, 25 g of faeces was weighed from each sample for the extraction of larvae using modified Baermann technique [12]. The faeces were enclosed in gauze fixed on the string rod and submerged in a clean glass tube filled with warm water. The whole apparatus was left for 2 to 3 h. The larva leaves the faeces and migrates through the gauze and settles at the bottom of the glass. After siphoning off the supernatant, the sediment was examined under the low power of the microscope [15]. All the area under the cover slip was thoroughly and uniformly searched for the presence of lungworm larvae [16]. A drop of 1% iodine solution was used to immobilize the larvae for species identification. If larvae were present under the microscope, small amount of specimen transferred to low power magnification of the compound microscope for morphological identification of lungworm larvae [17,18].
Post mortem examination: Those of samples used for coprological examination also used for postmortem examination which means 500 samples (240 cattle and 260 sheep) were collected for postmortem examination of adult lung worms and the identification of the species involved. The species, age, and body condition of slaughtered animals at Gondar Elfora abattoir were labeled. The lungs were palpated for presence of metastrongyloid nodules, which were usually grayish white in color. The air passages were opened starting from the trachea down to the small bronchi with fine blunt pointed scissors, to detect parasites [4]. If the nodules are present, they are trimmed off and worms extracted from the tissue by gentle pressing of a small non-calcified nodule or part of large nodule between two glass slides and then carefully teasing the worm away from the tissue. The collected worms were identified and recorded. Microscopic examination and identification of lungworms was performed using the features. D. filarial was occurred in the trachea, bronchi and bronchioles of sheep. This lungworm is slender, thread like nematodes, white in color with knob on head. Muellerius capillaries were occurred in the lung (bronchi, bronchioles and alveoli). This lungworm is small hair like with bent tail. Protostrongylus rufescens adults were found within the bronchioles, grey reddish in color and have wavy tail. The collected Parasites were examined using a compound microscope [7].
Data management and analysis
The data were entered and managed in Microsoft office excel work sheet. The analysis was conducted using SPSS version 20. Prevalence of lung worms were expressed as percentage with 95% by dividing the total number of animals positive to lung worms to the total number of animal examined. In all the analysis, confidence level was held at 95% and 5% level of significance [13].
Coprological examination
Prevalence of lungworm infection in cattle and sheep: In this study, a total of 500 cattle and sheep (240 cattle and 260 sheep) fecal samples were collected from the Gondar Elfora abattoir after the animals arrived for the purpose of slaughter. The overall lungworm prevalence of cattle and sheep recorded was 3.75% and 7.31% respectively. Out of the total 500 animals examined for the prevalence of lungworm infection in cattle, the age group (young and adult) was 7.04%, and 2.37%, and body conditions (poor, medium, and good) were 13.64%, 3.3%, and 0% were recorded respectively. Whereas, in sheep, with regard to age group, young 6.8% and 7.9% and with body conditions, poor 16.2%, medium 5.35% and good 8.33% positive for lung worm infection. In this study, age has no a significant variation (p >0.05) in cattle. Body condition of cattle has a statistically significant variation (p <0.05). But in both the age group and body condition in sheep, lung worm infection has no stastically significant variation (p >0.05) (Table 1).
| Species | Risk Factors | Total No. Examined |
Total no. of Positive (%) |
Total Prevalence (%) | X2 | P- value | |
|---|---|---|---|---|---|---|---|
| Bovine | Age | Young | 71 | 5 (7.04%) | 9 (3.75%) | 3.03 | 0.08 |
| Adult | 169 | 4 (2.37%) | |||||
| Body condition | Poor | 22 | 3 (13.64%) | 7.46 | 0.02 | ||
| Medium | 182 | 6 (3.3%) | |||||
| Good | 36 | 0 (0%) | |||||
| Ovine | Age | Young | 147 | 10 (6.8%) | 19 (7.31%) | 0.13 | 0.72 |
| Adult | 113 | 9 (7.9%) | |||||
| Body condition | Poor | 37 | 6 (16.2%) | 5.45 | 0.06 | ||
| Medium | 187 | 10 (5.35%) | |||||
| Good | 36 | 3 (8.33%) |
The overall prevalence of lung worm species in sheep: Under coprological examination, the overall prevalence of lungworm species in sheep recorded in this study were D. filarial, M. capillaries, and P. rufescens, with prevalence of 52.63%, 36.84%, and 10.53% of positive animals, respectively, in decreasing order (Table 2).
| Lung Worm Spp. | No. of positive | Prevalence (%) | X2 | P-value |
|---|---|---|---|---|
| D.filaria | 10 | 52.63 | 69.8 | 0.026 |
| M. capillaries | 7 | 36.84 | ||
| P. Rufescens | 2 | 10.53 | ||
| Total | 19 | 100.00 |
Abattoir survey (Post-mortem examination)
Out of a total of 240 cattle examined both coprologically and postmortem, 3.75 percent (9/240) and 4 (1.67%) respectively were positive for D. viviparous lung worm species infection.
Prevalence of cattle and sheep lungworm infection: A total of 240 cattle and 260 sheep were examined. The overall prevalence of lung worm infection in cattle with respect age group was young 4.23% than adult 0.59%. Concerning the body condition score the finding were recorded in poor, followed by medium and good body condition (4.55%, 1.65% and 0.00%) respectively. Age group in bovine has a statistically significant variation (p <0.05) (Table 3). In ovine species the age wise prevalence was higher in adult than young with the percentage of (7.48% and 8.85%) respectively. Regarding to body condition, higher good body condition score followed by poor and medium (13.89%, 8.11% and 6.95%) respectively (Table 3).
| Species | Risk factors | Total no. Examined |
Total no. of Positive (%) |
Total Prevalence (%) | X2 | P-value | |
|---|---|---|---|---|---|---|---|
| Bovine | Age | Young | 71 | 3 (4.23%) | 4 (1.67%) | 4.028 | 0.045 |
| Adult | 169 | 1 (0.59) | |||||
| Body condition | Poor | 21 | 1 (4.55%) | 1.72 | 0.42 | ||
| Medium | 179 | 3 (1.65%) | |||||
| Good | 36 | 0 (0.00%) | |||||
| Ovine | Age | Young | 147 | 11 (7.48%) | 21 (8.08%) | 0.16 | 0.69 |
| Adult | 113 | 10 (8.85%) | |||||
| Body condition | Poor | 37 | 3 (8.11%) | 1.96 | 0.38 | ||
| Medium | 187 | 13 (6.95%) | |||||
| Good | 36 | 5 (13.89%) |
The relative percentage of adult lung worm species extracted from sheep: Under postmortem examination, the overall prevalence of lungworm species in sheep recorded in this study were D. filarial, M. capillaries, and P. rufescens, with prevalence of 28.57%, 19.05%, and 9.53%, respectively, in decreasing order. Mixed infection was higher with the observed prevalence of 42.85% of positive animals (Table 4).
| Lung Worm Spp | No. of Positive | Prevalence (%) | X2 | P-value |
|---|---|---|---|---|
| D.filaria | 6 | 28.57 | 72.05 | 0.025 |
| M. capillaries | 4 | 19.05 | ||
| P.Rufescens | 2 | 9.53 | ||
| Mixed infections | 9 | 42.85 | ||
| Total | 21 | 100.00 |
Lung worm infection (verminous bronchitis, verminous pneumonia) is a chronic and prolonged infection caused by nematodes that affects the lungs of cattle and sheep. This disease results in substantial economic losses due to the reduction of growth rate, morbidity and mortality as the disease exposes animals to secondary bacterial infection [19].
In the current study, the overall prevalence of lungworm infection under coprological examination was 7.31% in sheep and 3.75% in cattle. The overall prevalence of cattle lungworm infection (3.75%) found in this study was very low as compared to the previous studies conducted by Radostits O, et al. [20] in Kembibit District with a prevalence of 10%, but this study was high as compared to the previous published research reported by Yihenew GA and Temesgen TY, et al. [21] in Gondar Town with a prevalence of 3.1%. The overall prevalence of sheep lungworm infection (7.31%) found by coprological examination was very low as compared to the previous studies conducted by Radostits O, et al. [20] in Kembibit District with a prevalence of 42%, [2] in the North Gondar Zone with a prevalence of 39.8%, and [2] in Gondar Town with a prevalence of 33.83%. Also, this finding was not high as compared to the previous published research. The possible reasons for the low prevalence in this study for both cattle and sheep could be attributed to the development of an open-air clinic, careful management, and increasing awareness of farmers to deworm their animals against parasitic infections in the study area, apart from geographical variations.
The overall prevalence of lungworm in cattle under postmortem examination (1.67%) found in this study was high as compared to the previous studies conducted by Yihenew GA and Temesgen TY, et al. [21] with the prevalence of (0%) and (1.5%) reported by Alemu Y and Merkel R [22] in Addis Ababa abattoir. In sheep, under postmortem examination, the overall prevalence of lungworm infection (8.08%) was low as compared to the previous studies conducted by FAO [2] in Gondar town by different restaurants, with a prevalence of 20.74%. The possible reason for such prevalence variation in both cattle and sheep might be due to animals purchased for slaughtering purposes coming from different market places and agro-ecological zones that favor the survival of the larvae of the lung worms, the methods used for the detection of the larvae, differences in humidity and temperature, season of examination, treatment condition, veterinary service altitude, probability of deworming, and rainfall differences in study type, that might affect the abundance of lungworms [8,23].
The prevalence of lung worm both in cattle and sheep was compared between animals of different age groups, under coprological examination. Though the age group result was not statistically significant, different prevalent rates in different age groups were found (p >0.05). Under coprological examination, the prevalence of lung worm infection, with regard to age group, was higher in young cattle (7.04%) than in adult cattle (2.37%). This result agrees with the previous reports conducted by Radostits O, et al. [20] in Kembibit District, <2 years higher than 2-4 years and >4 years, with a prevalence of 61.5%, 20.5% and 37.5% respectively, and [21] in Gondar Town, 1-5 years and >5 years, with a prevalence of 5.6% and 0% respectively.
The current result in sheep was higher in adult (7.9%) than young (6.8%), this result agrees with the previous result conducted in Kembibit District >3 years higher than 1-3 years with the prevalence of 37.5% and 20.5% respectively, [24] in and around jimma, 6-24 months of age higher than sheep in the age of <6 months with the prevalence of 27.0% and 23.0% respectively, [25] in Dangle District adult(1-3years) higher than young(<1year) with the prevalence of 16.4% and 15.6% respectively, [26] in south Wollo zone >3years higher than 1-3years and <1 year with the prevalence of 69.01%, 41.67% and 19.84% respectively, [2] in North Gondar zone >4 years higher than 6months -4years followed by ≤ 6months with the prevalence of 31.5%, 30.8% and 11.6% respectively, [2] in Gondar Town >4years higher followed by 6 months – 2years and 2-4years with the prevalence of 37.75%, 32.93% and 28.57% respectively.
But this result disagree with the previous reports conducted by Radostits, et al. [20] in Kembibit District with the prevalence of <1 year higher than followed by >3 years and 1-3 years with the prevalence of 61.5%, 37.5% and 20.5% respectively, [24] in and around jimma 6-24months higher than>24 months with the prevalence of 27.0% and 18.3% respectively and <6 months higher than >24 months of age with the prevalence of 23.0% and 18.3% respectively, [2] North Gondar Zone 6months -2 years (30.8%) higher than 2-4 years (26.5%). The prevalence difference among the different age groups of the cattle and sheep might be associated to the development of acquired immunity in adult animals from previous exposure which makes them to have the lowest infection, lowest prevalence and this might be related to the variation for disease exposure among study groups in the different study areas, which is also supported by Mulate B and Mamo M [15].
An attempt was made to see the influence of body condition on the overall prevalence of lung worm infection in cattle was found statistically significant (p<0.05) but in sheep not statistically significant (p>0.05). Under coprological examination, the prevalence of cattle lung worm infection in poor body condition (13.64%) was found to be too high when compared with animals in medium (3.31%) and good body condition (0.00%). This result agrees with the previous reports conducted by Radostits O, et al. [20] in Kembibit district on poor (20%), medium (10.34%), and good (4.35%), [24] in and around Jimma, poor (29.9%), medium (22.7%), and good (13.6%), and [13] in and around Jimma, poor (48%), medium (32%), and good (19%). This difference might be due to the fact that poor body-conditioned animals are easily exposed to infectious and non-infectious diseases, whereas medium and good bodyconditioned animals are not. Because of these and other related reasons, their immune systems become suppressed and less competent in getting rid of lungworm infections, which increases the degree of pasture contamination in an extensive system of management, which increases the degree of exposure. Furthermore, the response of a lungworm infection varies widely depending on the nutritional status, the age of the host, and the number of larvae ingested. And also, the possible reason could be due to immune suppression in poor body condition, concurrent infection by other parasites, means of examination and GIT helminthes simultaneously as reported by Thrusfield M [27].
In the current study, in cattle, the only lung worm species, D. viviparous, was detected, both under coprological and postmortem examination. But in sheep, under coprological examination, D. filarial was the most prevalent (52.63%) of the total positive samples examined, followed by Muellerius capillaries (36.84%) and Protostrongylus refuses (10.53%). This result agrees with the previous report conducted by Kahn CM [28] in selected areas of Dale District, with the prevalence of (26%, 18% and 10%), [29] in and around Hawassa, with the prevalence of (6.77%, 3.71% and 4.69%), [24] in and around Jimma, [30] with the prevalence of (35.42%, 7.55% and 0%) respectively. But this result disagrees with the previous report conducted by Radostits O, et al. [20] in kembibit district, Protostrongylus rufescens higher than Muellerius capillaries, having the prevalence of (9.4% and 6.5%) respectively. But under post mortem examination, mixed infection was higher followed by Dictyocaulus filarial, Muellerius capillaries and Protostrongylus rufescens with prevalence of 42.85%, 28.57%, 19.05% and 9.53% respectively. This result agrees with the previous studies conducted by Kahn CM [28] in Yirgalem Municipal abattoir with the prevalence of (26.6%, 18%, 7.1% and 5.3%) respectively. But this result disagrees with the previous report conducted by Habte D and Simeneh A. [26] in Dessie municipal abattoir, M. capillaries was higher infection followed by mixed infection with two or three species, D. filarial, and P rufescens with the prevalence of (23.48%, 8.18%, 8.18% and 5.87%) respectively.
The variation might be in the life cycle of the lungworm species. For instance, D. filarial has a direct life cycle and takes less time to reach the infective stage, and after ingestion, larvae can appear in feces within 5 weeks [31]. Whereas P. rufescens and M. capillaries have an indirect life cycle that requires longer time and a wet or rainy season to complete their complex life cycle in the presence of suitable intermediate hosts that create favorable conditions for sporadic distribution, they stand at the least prevalent rank. On the other hand, the low prevalence rate of P. rufescens, whose intermediate host range is restricted to certain species of snail, is lower than the rest, despite having the same geographic range as [27,32-35].
The result of the current study revealed that, lungworm infection is one of the prevalent diseases of cattle and sheep at Gondar Elfora abattoir. The prevalence of lung worm infection was higher in those cattle with poor body conditions than in those with medium and good body conditions. Dictyocaulus viviparous in cattle and Dictyocaulus filarial in sheep was the dominate lung worm species in the study area. It can also be concluded that the infection caused by lungworms was common in the study area and it was an important health problems of cattle and sheep to cause serious economic loss. Since the disease is prevalent and economically important, to take appropriate control and prevention measures, animals brought to Gondar Elfora abattoir for slaughter supplier/merchants should be recorded the origin and management system of animals.
All abattoir workers sincerely acknowledged for their willingness and committed support for success of this work.
Baye wodaje and Samrawit melkamu have conceived study, collected samples and processed in laboratory, analyzed data and wrote the draft manuscript. Both have read and approved the final manuscript.
Not applicable.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.
The authors declare that they have no competing interests.
Journal of Animal Health and Behavioural Science received 38 citations as per Google Scholar report