Research Article - (2022) Volume 13, Issue 8
Received: 03-Aug-2022, Manuscript No. JVST-22-71131;
Editor assigned: 11-Aug-2022, Pre QC No. P-71131;
Reviewed: 17-Aug-2022, QC No. Q-71131;
Revised: 23-Aug-2022, Manuscript No. R-71131;
Published:
30-Aug-2022
, DOI: 10.37421/2157-7579.2022.5.142
Citation: Amenu Misgana, Bedu Hussen, Neggasa Tolesa and Benti Tefere. “Sero Prevalence, Associated Risk Factors and Knowledge, Attitude and
Practice of Farmers towards the Contagious Bovine Pleuro Pneumonia in Selected Districts of West Arsi Zone of Oromia Regional State.” J Vet Sci Techno 13 (2022): 142.
Copyright: © 2022 Bedu H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contagious bovine pleuropneumonia (CBPP) remains a huge threat to cattle production in sub–Saharan African countries like Ethiopia. A cross sectional study was conducted between September, 2020 to June, 2021 to determine seroprevalence, to identifying animal and herd level risk factors and to estimate knowledge, attitude and practice (KAP) of farmers toward CBPP disease, in districts of Kofele and Siraro. A total of 384 animals were randomly selected with no history of vaccination against CBPP and serum samples were collected and tested by competitive ELISA. Questionnaire survey from 74 cattle owner was collected and data obtained from both serological and questionnaire surveys were analyzed by using SPSS software version 25. The results indicated that, the overall animal and herd level seroprevalence of CBPP were 16.14% (62/384) and 43.24% (32/74) respectively. Among the potential risk factors analysed by Univariate logistic regression analysis, those p-value less than 0.25 were analysed by Multivariate logistic regression and this showed that in age group sampled adult cattle (OR=1.642, CI95%:1.110-2.43, P=0.021) were 1.64 times to be affected than young. Animals which had history of respiratory problems (OR=2.5, 95% CI: 1.5-4.1, P=0.000) were 2.5 times more likely to be seropositive than those hadn’t history of respiratory problems, poor body condition animals (OR=8.4, 95% CI: 1.5-10, P=0.001) were 8.4 times more likely to be seropositive than good body condition and cattle that found in lowland area (OR=2.6, 95%CI: 1.34-4, P = 0.001) were 2.6 times more likely to be affected by CBPP disease than animals found at highland area. As well as the likelihood of getting risk of infection with CBPP disease of female (OR=2.6; 95% CI: 1.45-3, P=0.014) is 2.6 times than male. This study showed that the overall prevalence of CBPP was high, so appropriate implementation of appropriate prevention and control is needed. The questionnaire result indicates majority of farmers were practicing poor animal husbandry that created favourable conditions for the distribution of the disease in the community. Therefore, to deal with CBPP disease, further farmers should be made aware of about the disease and its importance through veterinary extension education.
Contagious bovine pleuropneumonia (CBPP) • Knowledge • Attitude and Practice (KAP) • Kofele, Seroprevalence, Siraro, Risk factor
Background
Ethiopia is heavily depending on agriculture sector which play a major role in overall economic development. Among the agricultural sectors, livestock is the one which is ranked first in Africa and tenth in the world [1]. Livestock provides a livelihood for 65% of the total population [2], and 80% of the rural population of the country and contributes 15-17% of Gross Domestic Product (GDP), 35- 49 % of agricultural GDP and 37-87% of the household incomes [3,4]. Therefore, an improvement of this sector has the potential to contribute significantly to national income and to the welfare of the majority of rural families [5]. The livestock species that are found in Ethiopia, cattle are the most important to the GDP of the country [6]. Cattle are used as source of draught power for the rural farming population, supply farm families with milk, meat, manure, and also as source of cash income, playing a significant role in the social, and cultural values of the society [1,7,8].
According to recent estimation, Ethiopia has 56.71 million of cattle population [9] and this figure clearly indicated how much cattle are key important sectors of agriculture which take part in a potential pathway out of poverty of many, particularly for rural farmers [10,11]. Regardless of the large number of cattle we have, the sector is characterized by low productivity. Income derived from this sector of agriculture could not bear significant role in the development of the country’s economy due to many constraints mostly diseases. Cattle disease problems were extremely exacerbated by drought, concentration of livestock at watering points and dry grazing grounds combined with reduced resistance, intensifies the spread of contagious and parasitic diseases which often cause higher losses than the forage or water shortages [12]. Ethiopia was ranked highest among Sub-Saharan countries in livestock disease burden [13], deaths estimated for Ethiopia due to various diseases were 3.23 million cattle, 4.37 million sheep and 4.90 million goats (CSA, 2015).
Contagious Bovine Pleuro Pneumonia (CBPP) is a highly contagious disease of cattle that is caused by Mycoplasma mycoides subsp. mycoides small colony (MmmSC) [14,15]. It has great potential for rapid spread and causes major impact on cattle production. The disease is usually spread by movement of animals across international boundaries with devastating consequences on cattle, particularly in severe outbreaks [16]. Transmission occurs mainly by aerosol through close contact with infected animals within herds and from herd to herd through direct contact and repeated contact between sick and healthy animals, and occasionally from latent carriers intermittently shedding Mycoplasma organisms from sequestrated lung lesions [17].
The disease is manifested by anorexia, fever and respiratory signs such as dyspnoea, polypnoea, cough and nasal discharges in cattle [18,19]. The principal route of infection is inhalation of infective droplets of diseased animals. Factors such as extremes of age, stress and concurrent infections may predispose to tissue invasion [20]. It is considered to be a disease of economic importance because of its high mortality rate, production loss, increased production cost due to cost of disease control, loss of weight and working ability, reduced fertility, and loss of cattle trade [21,22]. Due to its potential for rapid trans-boundary spread and its high economic impact, OIE declared as one of the most serious contagious animal diseases and listed on the group of notifiable animal diseases of high socio-economic impact and regarded as major trans-boundary animal disease (TADs) (FAO, 2002).
CBPP is one of the great plagues that continue to devastate the cattle herds on which so many people are dependent in Africa and considered as the most serious infectious animal disease affecting cattle of the continent [23,24]. The disease spread alarmingly during the 1990, infecting several countries previously free from the disease and causing greater losses [25,26]. In recent years, the disease has emerged from areas where it has been persisting in endemic form to re-invade other areas from which it had previously been eradicated. In addition to these newly infected areas, the endemic areas are experiencing an increasing in the incidence of CBPP [27].
With the imminent eradication of rinderpest, CBPP has becoming the most important cattle disease that hinders livestock development of Ethiopia. This is mainly caused due to the interruption of the consecutive yearly blanket vaccinations with combined rinderpest. Since 1992/93 which had certainly contained the disease to a relatively low level during the past years by reducing the susceptible bovine population. However, CBPP is now re-emerging as one of the most economically important diseases which have impact on livestock production. Poorly understanding of pathogenesis, relatively ineffective vaccines or with adverse effects and poor diagnostic assays were further rise the impact of the disease in the country [28]. Ethiopia is experiencing the largest number of cattle deaths, and reduction in cattle products under both endemic and epidemic conditions of CBPP compared to the other African countries probably due to large cattle population [29]. Therefore, CBPP was considered as one of the most important cattle diseases and impute impact to livestock development of the country [30].
In most continents, control strategies are based on the early detection of outbreaks, control of animal movements and stamping-out policy. However, in Africa control of the disease is only based on vaccination (T1/44 or T1SR) and antibiotic treatment [31]. However, the consequences of antibiotic treatments in terms of clinical efficacy, emergence of resistant strains and persistence of chronic carriers have not been evaluated yet [32]. But currently research work has shown that antibiotic treatment of cattle may greatly reduce the transmission to healthy contacts but this requires treatment of all affected cattle [33]. Despite vaccination has been considered as a strategy for the control of CBPP in Ethiopia, the disease still persists in several regions of the country and its incidence increasing from year to year. This is, mainly due to lack of effective vaccine, irregular and low coverage of vaccination, lack of livestock movement control, and absence of systematic disease surveillance and reliable data [34].
Knowing the extent of diseases distribution like CBPP is valuable since used as an input for development of optimum prevention and controlling strategies and that will ultimately assist in poverty alleviation by improving the productivity of the sector. Therefore, to carry out an effective control of the disease, prerequisites such as understanding of the epidemiological sce nario of the disease and farmer’s knowledge, attitude and practices towards the disease should be well-known. Currently there were several studies have been undertaken Ethiopia since 1995 on CBPP as a country with prevalence of 0.4%-96 [35] respectively.
Statement of the problem
Countries in East Africa reported 66% of the total outbreaks (58% in Ethiopia and Tanzania and 8% in other countries). CBPP is endemically maintained all over the country with 80% morbidity and greater than 50% mortality (CFSPH, 2015). In general, CBPP has been causing significant economic losses on the agricultural sector and the national economy of Ethiopia. Over the last decades, the country has lost a substantial market share due to frequent bans by the Middle East countries (OIE, 2009). In present day of Ethiopia, there is a national drive to alleviate the existing food deficit by devising different agricultural strategies including improvements of the productivity of livestock sector by controlling some of the major diseases through regular monitoring to achieve transformation plan [36].
The previous studies were stressed mainly only on seroprevalence study, whereas there were no reported documents on farmer’s knowledge, attitude and practices towards the disease to date in the country in general and southern Oromia in particular. West Arsi Zone possesses huge livestock resource in which Cattle play a significant role in supporting the people’s livelihood in the area. The exact picture, dynamics and distribution of CBPP disease in the area is not well documented. Knowledge and understanding of the epidemiological profile of animal diseases is critical for evaluating and addressing the veterinary health needs of the livestock population of the area. Hence, there is scarcity of well documented information on the current status and distribution of the disease in the area. Beside of this, in this area there was no systematic study conducted to look into the status of this important disease and there were different animal diseases of unknown etiologic agent which are often suspected by field veterinarian. So, I have initiated to study this problem and lastly, I decide the work to be under taken with following objectives;
Objectives
General objective: Assessing the knowledge, attitude and practice (KAP) of farmers towards the disease and estimating seroprevalence and associated risk factors of CBPP in Kofele and Siraro, west Arsi Zone, Oromia Regional State, Ethiopia.
Specific objectives:
1. To assess the knowledge, attitude and practice of farmers towards the CBPP disease in study area.
2. To Estimate the Sero-Prevalence of Contagious Bovine Pleuro pneumonia in study area.
3. To identify the associated risk factors for the occurrence of Contagious Bovine Pleuro pneumonia in study area.
Description of study area and study animal
The study was carried out in two selected districts of Kofele and Siraro which were selected from West Arsi zones of Oromia regional state, Southern Ethiopia. West Arsi is one of the zones of Oromia Region in Ethiopia. This Zone has a total population of 1,964,038, of whom 973,743 are men and 990,295 women. 272,084 or 13.85% of population are urban inhabitants. A total of 387,143 households were counted in this Zone, which results in an average of 5.01 persons to a household, and 369,533 housing units. The two largest ethnic groups reported in West Arsi were the Oromo (88.52%) and the Amhara (3.98%); all other ethnic groups made up 7.5% of the population. Afan Oromo is spoken as a first language by 87.34% and 6.46% spoke Amharic; the remaining 6.2% spoke all other languages. The majority of the inhabitants were Muslim, with 80.34% of the population, while 11.04% of the population Orthodox and 7.02% of the population Protestant [37].
Kofele is one of the Districts in the Oromia Region of Ethiopia (Figure 1) and the district has 38 rural Kebele and 3 towns. Kofele is bordered on the south by the Kokosa, on the west by the Southern Nations, Nationalities and People’s Region, on the northwest by the Shashamene district, on the north by Kore, on the east by Gedeb Asasa, and on the south east by Dodola. The altitude of this woreda ranges from 2000 to 3050 meters above sea level; Mount Duro is the highest point. Rivers include the 35 kilometers of the Anjelo, 30 kilometers of the Totalamo, and 35 kilometres of the Ashoka, all of which are tributaries of the Shebelle River. A survey of the land in this District shows that 30% is arable or cultivable, 29% pasture, 2.9% forest, and the remaining 38.1% is considered swampy, mountainous or otherwise unusable [37].
The district is also endowed with a significant number of domestic animals; 97,560 cattle, 89,240 are indigenes and 8320 crossbreds, 39,206 sheep, 2107 goats, 229 equines, 75,450 poultry and 5786 honeybee colonies are found in the district [38]. The 2016 national census reported a total population for this District is 178,950, of whom 89,729 were men and 89,221 were women; 15,448 or 8.63% of its population were urban dwellers. The majority of the inhabitants were Muslim, with 94.32% of the population, while 2.88% of the population is Orthodox and 1.94% of the populations were Protestant [37].
Siraro is one of the woredas in the Oromia Region of Ethiopia and is found in West Arsi Zone in the Great Rift Valley (Figure 2). Siraro is bordered on the south and west by the Southern Nations, Nationalities and People’s Region; on the north by Shala, and on the east by Shashamene Zuria; its western boundary is defined by the course of the Bilate River. The livestock populations of the district were: Cattle (190,166), Sheep (35, 952), Goat (36,512), Poultry (193,155), Horse (12,249), Donkey (16,402) and Mules (1,196) [37]. The administrative centre of this woreda is Loke and is located on geographical coordinates 9°04'59.9" N and 36°49'59.9" E. The altitude of this woreda ranges from 1500 to 2300 meters above sea level [39].
The agroecology of the area is 7.53% highland, 74.2% midland and 18.7% low land type. It experiences a bimodal pattern of rainfall with the main rainy season extending from June to September (84% of rain is expected) and short rainy seasons from March to May. The area receives rainfall ranging from 1000-2400 mm with mean annual rainfall of 1700 mm. The minimum and maximum temperatures of the district were 10.90 C and 33.90 C, respectively.
Study design
The cross-sectional study design was conducted from September 2020 to June 2021 in two districts of Kofele and Siraro from west Arsi zone, Southern Ethiopia. The purpose of the study was fully explained to the selected cattle owners. Before conducting the study to have owner’s agreement and questionnaire interviews were conducted during a face-to-face interview with the study participants. From each interviewed household, animal’s blood sample collections were performed. The overall animal and herd level seroprevalence of CBPP were estimated as well as the associated potential risk factors were identified.
Sample size and sampling method
Sample size determination: The sample size required for blood collection was determined using the formula given by Thrusfield (2007). Since there were no recent previous reports of prevalence of CBPP disease in the study area we use an expected prevalence of 50% with 5% the desired absolute precision and 95% confidence interval. Therefore, the sample size was calculated by the formula:
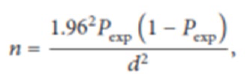
Where,
n= is number of animals that was sampled; P =is the expected prevalence of the disease in 50% and d= is precision level (5%). This gives total sample size of 384 (three hundred eighty-four) cattle. Therefore 384 cattle were sampled, in which 192 cattle fromKofele and 192 cattle from Siraro.
An estimated sample size for households that participated in the questionnaire survey was calculate by dividing the total sample size (n=384) by the number of animals sampled within each herd or household (7) and given an estimated of 55 households for inclusion in the questionnaire survey [40]. However, due to the inclusions of households that had less than seven animals, the total sample size of households was increased to 74 [41]. Therefore, in this study a total of 74 households or herds were selected. From these 38 herds from Kofele (17 from Wameny Abosa and 21 from Tulubako PAs) and 36 herds from Siraro (18 from each Shasha and Gayo) were selected.
Sampling methods: Multi stage sampling method was used in which Zone, districts and Peasant Associations was selected purposively based on easily accessible and densely populated are of cattle. Kofele has 38 Pas while Siraro has 26 PAs. From this district 2 PAs was selected. From PAs of each district heard/households were selected purposively in which those households having greater than 2(two) animals were selected as heard. From house hold having 2-7 animals all animals were selected but for those having greater than 7(seven) animal we select only seven animals by random sampling method. In this case the owner of study animals was recognizes each cattle owned by name, thus, animals were randomly sampled using the name of animals as ID number and blood samples collections were conducted. Since between cluster variance of CBPP disease in the area is unknown a simple random Samling method was applied to calculate number of animals to be included in each heard.
Data collection
Questioner survey: A questionnaire survey was made to evaluate farmer’s knowledge, attitude and practice (KAP) towards the general overview of cattle respiratory health problems with special emphasis to CBPP. Therefore, a detailed questionnaire (Appendix 3) was prepared in English before being translated into Afan Oromo. Questionnaire survey was administered by faceto- face interview with the owner of animals using local language which is Afan Oromo. Information that contained in the body of developed questionnaire were: general household characteristics; cattle herd size and structure to ascertain farmers knowledge, attitude and practices, general overview of cattle’s respiratory disorders within the herds, farmers assumption related to the factors that causes the disorders, any infectious diseases known by farmers, major signs of CBPP disease that recognized by farmer’s, any respiratory infectious diseases that are known by farmer’s, possible transmissions methods of CBPP or any contagious diseases, economic importance and farmer’s routine cattle management practices that are associated with prevention and controlling methods of contagious infectious diseases like CBPP were the major issues that included in the questionnaire.
Blood sample collection: Animals were restrained by owners in the cattle crush made by the help of DA’s (development agency) and blood samples of animals were collected for diagnostic test. Therefore, from each sampled animal about 10 ml of whole blood was collected from the jugular vein of the animals using disposable plain vacutainer tubes and needles. After collection, the samples were properly labelled and left for 24 hours at room temperature to allow clotting and after which the sera were transferred to serum tubes and kept at -20 °C at Asella regional Veterinary laboratory until they were tested at NAHDIC (National Animal Health Diagnostic and Investigation Centre) department of bacterial serology laboratory. Then serum samples were analysed using competitive ELISA (cELISA) for the detection of contagious bovine pleuropneumonia antibodies according to OIE standard OIE (2014) OIE terrestrial manual.
During blood sample collection variable that considered as risk factors are altitude (high land and low land); age (grouped into two which is 6 months to 3 years old called young and greater than 3 years old called adult) based on owners information; sex (male and female); herd size (grouped in to three, since the interviewed minimum and maximum number of herd sizes ranges 2-100 categorized as( 2-5 called small herds, 6-14 called medium herds and 15-100 large herds); previous history of respiratory disorder and body condition score (BCS) (characterized based on [42] principles, was recorded.
Laboratory test (Competitive ELISA)
Competitive enzyme-linked immunosorbent assay (ELISA) was conducted as recommended by CIRAD-UMR15 (France) and is based on a monoclonal anti-MmmSC antibody named Mab 177/5 as previously described (LeGoff and Thiaucourt 1998). The sensitivity and specificity of the diagnostic test used were, respectively, 98% and 97%. Serum samples were mixed with specific monoclonal antibody (Mab 117/5) in a dilution plate and incubated with gentle agitation for 37 °C for 1 h, then transferred into the MmmSC-coated microplate.
After washing, anti-mouse IgG serum–conjugated horse radish peroxidase was added. After a series of washings, the horse radish peroxidase substrate (TMB) was added forming a blue compound that turned yellow when the reaction was stopped. The optical density was read in an ELISA reader at 450nm and the cut-off point was calculated to validate the results. All sera with percentage inhibition (PI) > 50% were considered positive. Sera with PI between 40% and 50% were considered doubtful and those sera with PI less than 40% were negative.
Competitive enzyme-linked immune sorbent assay (ELISA) was conducted as recommended by CIRAD-UMR15 (France) and is based on a monoclonal anti-MmmSC antibody named Mab 177/5. Micro plates are coated with MmmSC purified lysate. Samples to be tested are premixed with a specific monoclonal Mab 117/5 in a separate plate (prelate) and content of the preplate is transferred into the coated microplate. Any MmmSC specific antibodies present in the sample was form an immune-complex with MmmSC antigen coated on the microplate, competing with Mab 117/5 for the specific epitopes. After washing away unbound material, an anti-mouse antibody enzyme conjugate is added. In presence of immune-complex between MmmSC antigen and antibodies from the sample, Mab 117/5 cannot bind to its specific epitopes and the conjugate is blocked from binding to Mab 117/5. Conversely in the absence of MmmSC-Antibodies in the test sample, Mab 117/5 can bind to its specific epitopes and the conjugate is free to bind to Mab 117/5. Unbound conjugate is washed away and enzyme substrate (TMB) is added.
In presence of the enzyme, the substrate is oxidized and develops a blue compound becoming yellow after blocking. Subsequent colour development is inversely proportional to the amount of anti-MmmSC antibodies in the test sample. The result is expressed in percentage of inhibition (PI) by comparing the optical density in the test well with the optical densities in the mAb control wells.
Data management and analysis
Data obtained from both serological tests and questionnaire surveys was entered and stored in Microsoft (MS) Excel spread sheet program. This data was analysed by SPSS software programs version 25. The total Seroprevalence was calculated by dividing the number of c-ELISA positive animals by the total number of animals tested. And herd level seroprevalence was calculated by dividing the number of c-ELISA positive heard (if one animal is positive from the heard, the heard is considered as positive) by the total number of heard sizes.
Initially Univariable logistic regression was used to assess the relationship between CBPP antibody seroprevalence, to estimate animal level and heard level seroprevalence and to assess individual risk factors for CBPP seropositivity. The risk factors included were breed, sex, age, herd size, history of respiratory disease, altitude, BCS, district and Peasant Association. Which were identified at p< 0.05. For those variables which are significant at p < 0.025 were subjected to multivariable logistic regression. The strength of association between the risk factors and the occurrence of the disease was assessed using Odds Ratio (OR).
Overall prevalence of contagious bovine pleuropneumonia
In the current study the sera were collected from two districts of Kofele and Siraro. A total of 384 cattle among 4 peasant association were examined by using competitive enzyme linked immune sorbent assay (c-ELISA). The result of this examination reveals that 62 animals were appeared positive for disease among sampled animals.
The overall sero-prevalence of Contagious Bovine Pleuropneumonia in the study area was 16.14 % (62/384). From two districts, the result indicates that in Kofele it shows 8.85% (17/192) and in Siraro it is 23.4% (45/192). This shows that highest sero-prevalence of CBPP was observed in the Siraro (23.4%) and the lowest sero-prevalence (8.85%) was observed in Kofele.
From the four-peasant association the result indicates that 8.33% (8/96), 9.33% (9/96), 20.8% (20/96) and 26.04% (25/96), animals were positive for the disease in Wameny-Abosa, Tulubako, Shasha, and Gayo respectively. The highest sero-prevalence of CBPP was observed in Gayo (26.04%), whereas the lowest sero-prevalence (8.33%) in Wameny-Abosa peasant association of Kofele district. There is significant variation at (p<0.05) of contagious bovine pleuropneumonia sero-prevalence among peasant association shown in Table 1.
| Study area | Categories | No of examined | Number of Positive | Prevalence | OR (95% CI) | p value |
|---|---|---|---|---|---|---|
| Districts | Kofele Siraro |
192 192 |
17 45 |
8.85 23.4 |
1 4.3(2.5-11) |
- 0.022 |
| Pas | Wameny Abosa | 96 | 8 | 8.33 | 1 | - |
| Tulubako | 96 | 9 | 9.33 | 2.8(2.6-12) | 0.043 | |
| Shasha | 96 | 20 | 20.8 | 3(1.4-8) | 0.036 | |
| Gayo | 96 | 25 | 26.04 | 4.6(1.8-20) | 0.024 | |
| Total | 384 | 62 | 16.14 |
Association of risk factors with the occurrence of CBPP: Host related potential risk factors like Herd size, Age, Body condition, Altitude, sex, HRD and Breed were studied its association with contagious bovine pleuropneumonia sero status. In this study, there was statistically significant association among age, body condition, altitude, sex, and HRD, at which (p<0.05) but there was not statistically significant in herd size and breed categories (p>0.05). The study shows that there was (18.81%) seropositivity in adult and (13.18%) in young, (29.45%) in poor body condition (12.41%) in medium body condition and (5.45%) good body condition, (23.43%) in lowland and (8.85%) in highland. As well as (21.29%) was observed in medium herd size and (11.9%) small herd size and (23.90%) in animals with history respiratory disorder (7.26%) healthy animals (Table 2).
| Variables | Categories | No examined | Number of Positive | Prevalence | OR (95% CI) | p value |
|---|---|---|---|---|---|---|
| Herd size | Small | 126 | 15 | 11.9 | 1 | |
| Medium | 108 | 23 | 21.29 | 0.6(0.3,1.4) | 0.222 | |
| Large | 150 | 24 | 16 | 1.4(0.7, 3) | 0.322 | |
| BCS | Good | 110 | 6 | 5.45 | 1 | |
| Medium | 145 | 18 | 12.41 | 6.9(2.-25) | 0.010* | |
| Poor | 129 | 38 | 29.45 | 8.4(1.2-10) | 0.000* | |
| Altitude | Highland | 192 | 17 | 8.85 | 1 | |
| Lowland | 192 | 45 | 23.43 | 1.9(1.3, 2.8) | 0.009* | |
| Breed | Local | 309 | 51 | 16.50 | 1 | |
| Cross | 75 | 11 | 14.66 | 1(0.5,2.3) | 0.898 | |
| Sex | Male | 175 | 15 | 8.57 | 1 | |
| Female | 209 | 47 | 22.48 | 2.6 (1.3- 5.2) | 0.012* | |
| HRD | No | 179 | 13 | 7.26 | 1 | |
| Yes | 205 | 49 | 23.90 | 2.5(1.5,4) | 0.000* | |
| Age | Young | 182 | 24 | 13.18 | 1 | |
| Adult | 202 | 38 | 18.81 | 1.6(1, 2.4) | 0.021* | |
| Total | 384 | 62 | 16.14% |
Herd level Seroprevalence of CBPP across the associated risk factors: Univariate Logistic regression analysis results showed that among herd level risk factors (District, PA, herd size and management system) that considered, only district, PA and herd size were statically significant effect on seropositivity (p<0.05). Such the OR of Siraro district is 3.9 (OR = 3.9, 95% CI: 1-14, P=0.031). While the PA of the Gayo is 4.6 (OR=4.6, 95%CI: 1-20, P=0.004) and Shasha is 3 (OR=3, 95%CI: 1.5-8, P=0.006) and in case of large heard size the OR is 3(OR=3, 95%CI: 1-8), P=0.003) (Table 3).
| Risk factors | Total herd | Positive (%) | OR (95% CI) | Std. Err. | p value | |
|---|---|---|---|---|---|---|
| Districts | Kofele | 38 | 13(34.21) | 1 | - | - |
| Siraro | 36 | 19(52.7) | 3.9(1.2-14) | 0.641 | 0.031 | |
| Wameny Abosa | 17 | 5(29.4) | 1 | - | - | |
| PAs | Tulubako Shasha Gayo |
21 18 18 |
8(38.09) 9(50) 10(55.5) |
2.8(0.6-12) 3(1.5-8) 4.6(1.8-20) |
0.74 0.445 0.756 |
0.165 0.006 0.004 |
| Herd size | Small | 25 | 4(16) | 1 | ||
| Medium | 30 | 14(46.6) | 2(1-6) | 0.78 | 0.148 | |
| Large | 19 | 14(73.6) | 3(1.5-8) | 0.48 | 0.003 | |
| Management system | Intensive | 3 | - | 1 | ||
| Semi intensive | 9 | 4(44.4) | 1.4(0.2-8.9) | 0.159 | 0.69 | |
| Extensive | 62 | 28(45.16) | 2.1(0.3-12) | 0.92 | 0.415 | |
| Total | 74 | 32(43.24) | ||||
Initially, univariate logistic regression was used to screen out all potential risk factors for statistical significance at (p <0.05) and variables that found statistically significant at (p<0.025) were subjected to multivariate logistic regression analysis with backward variable selection. The risk factors (District, BCS, Altitude, Sex, History of Respiratory Disorder and Age) that are statistically significant in univariable logistic regression analysis at (p<0.025) were included in the multivariate logistic regression model and analysed. Therefore, the final multivariable logistic regression model analysis result showed that District, BCS, Altitude, Sex, History of Respiratory Disorder and Age were statistically significant association with CBPP seroprevalence (P < 0.025) (Table 4).
| Variables | No exam | Prevalence (%) | Multivariable logistic regression OR (95%CI) (p value) | |
|---|---|---|---|---|
| BCS Good |
110 | 6(5.45) | 1 | - |
| Medium | 145 | 18(12.41) | 6.9(2-25) | 0.016* |
| Poor | 129 | 38(29.45) | 8.4(1.5-10) | 0.000* |
| Attitude Highland |
192 | 17(8.85) | 1 | - |
| Lowland | 192 | 45(23.43) | 2.6(1.34-4) | 0.001* |
| Sex Male |
175 | 15(8.57) | 1 | - |
| Female | 209 | 47(22.48) | 2.6(1.45-3) | 0.014* |
| HRD No |
179 | 13(7.26) | 1 | - |
| Yes | 205 | 49(23.90) | 2.5(1.5,4) | 0.000* |
| Age Young |
182 | 24(13.18) | 1 | - |
| Adult | 202 | 38(18.81) | 1.5(1.2-5) | 0.017* |
| Total | 384 | 62(16.14) | ||
Questionnaire result of Knowledge, Attitude and Practice (KAP)
Demographic structure, Herd size and characteristics of Respondents: Out of 74 households (82.1 %) were male and the rest (17.9 %) are females. Also, (88.1%) respondents were married. The ages of respondents were ranges 15 to 80 with mean of 41.2±19.1years old. Concerning educational status of the participants (42.9%) of them had no formal education while (11.9%) of them college (university) level educated and the mean of household size of participants was 7.7±2.9. Total number of cattle per household was ranging from 1-100. Out of a total 384 animals that 74 households had more than half of them were female cattle (72.8%) and (27.24%) were male. Similarly, (18.1%) cattle population were young (0.6- 3year), (81.9%) adults (> 3year) (Table 5).
| Parameters | Mean±SD (Range) | Frequency | Proportion (%) |
|---|---|---|---|
| Gender of respondents | |||
| Male | - | 59 | 82.1 |
| Female | - | 15 | 17.9 |
| Marital status | |||
| Single | - | 10 | 11.9 |
| Married | - | 64 | 88.1 |
| Educational Background | |||
| No formal education | - | 31 | 42.9 |
| Primary | - | 28 | 39.3 |
| Secondary | - | 5 | 6 |
| College or university | - | 10 | 11.9 |
| Age of respondents | 41.2±19.1(15-80) | ||
| House hold family size | 7.7±2.9(2-15) | 570 | |
| Herd Structure per household | |||
| Male | 3.5±2.4(0-12) | 259 | 27.24 |
| Female | 6.4±5.4(0-20) | 474 | 72.8 |
| Age | (0-60) | 1033 | |
| Young (0.6-3year) | 3.15±2.6(0-9) | 265 | 18.1 |
| Adult (>3 year) | 6.8±4.8(1-20) | 571 | 81.9 |
| Over all | 9.95± 6.9(1-25) | 74 |
Farmer’s knowledge and attitude related to CBPP disease in general:
Of the 74, 25(29.8%) of the respondents were knowledgeable with the cause of disorder while the majority of respondents had no any knowledge regarding to the factors that causes of respiratory disorders. (79.8%) of respondents were encountered respiratory problems of cattle, but (11.9%) of them recognized that the causes were due to infectious diseases while the majority of them had no reasons about the source of the problems and only (6%) of respondents had heard or knew CBPP while (94.1%) of the respondent hadn’t know (Table 6).
| Issue raised related to general overview of the respiratory disorder (RD) of cattle | Response Category (N=74) | |||
|---|---|---|---|---|
| Yes (%) | No(%) | Don’t know (%) | ||
| Have you encountered respiratory problems of cattle? | 57(79.8) | 6(7.1) | 11(13.1) | |
| Has your neighbour had any problems with cattle RD? | 29(40.5) | 17(20.2) | 28(39.3) | |
| Do you know factor that cause respiratory disorder? | 25(29.8) | 16(19) | 33(51.2) | |
| Do you think infectious disease can cause the RD? | 10(11.9) | 26(31) | 38(57.1) | |
| Have your several animal experienced respiratory problems simultaneously? | 49(64.3) | 25(35.7) | 0 | |
| Have you heard about CBPP disease? | 5(6) | 55(65.5) | 69(94.1) | |
| Have you encountered respiratory problems of cattle? | 57(79.8) | 6(7.1) | 11(13.1) | |
In this study the infectious diseases in general as well as respiratory diseases of cattle that known locally by farmers were assessed and the local names of the diseases were translated in to scientific or English name with the help of the nearby animal health worker of that area accordingly. As the result showed the predominant cattle diseases known by the respondents were trypanosomiasis (17.9%), blackleg (20.2%), pasturellosis (28.6%), and anthrax (10.3%), whereas dangila/ somba (51.2%) was the most known endemic respiratory disease of the area, however its scientific name couldn’t identify yet (Table 7).
| Issue raised to the respondent’s Local name of disease that known by farmer | Local name of disease that known by farmer | Scientific Name of the disease | Freq. (%) |
|---|---|---|---|
| Can you name any infectious diseases that found in your area or farm? | Hudha/Gororsisa | Pasturellosis | 24(28.6) |
| Cacabsa/Abagurba | Blackleg | 17(20.2) | |
| Aba-sanga/Dingetegna | Anthrax | 9(10.3) | |
| Gandi/Koksa | Trypanomosis | 15(17.9) | |
| Masa | FMD | 9(9.5) | |
| Infectious disease You know? | - | - | - |
| Dangila/ somba | CBBP | 38(51.2) | |
| Don’t know | Don’t know | 36(48.8) |
The proportion of each major signs of CBPP disease that known by respondents were: grunting during coughing or exhaling (66.7%), head extended coughing (72.6%), dilation of nostril and mucoid discharge (42.9%), swelled of throat and dewlap (52.4%), standing with back arched (40.5%), laboured & painful breathing (63.1%), standing with the elbows abducted (42.9%), chest pain (23.8%), frothy saliva at the mouth (58.3%), and polyarthritis on young (35.7%). Generally, in this study majority of the respondents were encountered or familiar with signs of CBPP disease (Table 8).
| Issue raised related to the major CBPP symptoms | Response Category (N=74) | ||
|---|---|---|---|
| Yes (%) | No (%) | Don’t know (%) | |
| Have you known or encountered any of the Following the major signs of CBPP disease? | |||
| Chest pain | 20(23.8) | 30(41.7) | 24(34.5) |
| Stand with the elbows abducted | 31(42.9) | 22(32.1) | 21(25) |
| Standing with back arched | 29(40.5) | 22(28.6) | 23(31) |
| Head extended coughing | 51(72.6) | 17(20.2) | 6(7.1) |
| Laboured & painful breathing | 43(63.1) | 14(16.7) | 17(20.2 |
| Grunting when exhaling (coughing) | 46(66.7) | 18(21.4) | 10(11.9) |
| Frothy saliva at the mouth | 39(58.3) | 19(22.6) | 16(19) |
| Dilation of nostril & mucoid discharge | 31(42.9) | 24(34.5) | 19(22.6) |
| Swelled throat and dewlap | 34(52.4) | 22(26.2) | 18(21.4) |
| Polyarthritis particularly on young | 26(35.7) | 24(32.1) | 24(32.1) |
Regarding knowledge of disease transmission, majority of the respondents hadn’t aware of the possible transmission methods of contagious diseases like CBPP. However, 65.5% and 59.5% of the respondents recognized as diseases transmitted through close contact with diseased animals and through coughing of infected animals, respectively. The Table 9 clearly summarizes the knowledge of respondents had regarding the possible transmission methods of contagious diseases like CBPP.
| Issue raised regarding to knowledge of disease transmissions methods | Response Category (N=74) | ||||
|---|---|---|---|---|---|
| Yes (%) | No (%) | Don’t know (%) | |||
| What are the possible transmission methods of CBPP disease or any contagious diseases among cattle of the following? | |||||
| Through contaminated feed or water | 30(35.7) | 23(27.4) | 21(36.9) | ||
| Transmitted through sexual contact | 13(15.5) | 31(36.9) | 30(47.6) | ||
| Close contact with diseased animal | 50(65.5) | 10(14.3) | 15(20.2) | ||
| Inhalation of infected droplets | 33(44) | 30(41.7) | 11(14.3) | ||
| Through contaminated fomites/objects | 30(36.9) | 25(34.5) | 19(28.6) | ||
| Can be transmitted through transplacental | 15(22.6) | 25(32.1) | 34(45.2) | ||
| Can be transmitted across long distance in air | 25(34.5) | 16(22.6) | 33(42.9) | ||
| Through saliva or urine of diseased animal | 10(17.9) | 34(40.5) | 30(41.7) | ||
| Through coughing of infected animal | 40(59.5) | 17(20.2) | 17(20.2) | ||
In the current study, majority of the respondents aware of the economic importance disease like CBPP for example (50%), (67.9%), (56%), and (59.5%), of respondents aware of as disease able to cause loss of body weight, loss of production, reduce working ability of cattle and mortality of cattle respectively. Regarding CBPP prevention and controlling methods, majority of the participants had basic knowledge on treatment of symptomized or diseased animal (75%) and vaccination (73.8%). Moreover, (31%) of the participants are aware of good management like decontamination of infected premises is used for disease prevention and controlling techniques. and isolation of new purchased animal from herd (22.6%) are used as disease prevention and controlling methods (Table 10).
| Issue raised related to economic importance and prevention and controlling | Response Category (N=74) | ||
|---|---|---|---|
| Yes (%) | No (%) | Don’t know (%) | |
| What is the economic importance of CBPP/ respiratory related diseases in cattle production? | |||
| Can cause mortality of cattle | 40(59.5) | 25(29.8) | 9(10.7) |
| Can cause loss of body weight | 37(50) | 25(35.7) | 12(14.3) |
| Reduced working ability of cattle | 43(56) | 25(33.3) | 6(10.7) |
| Reduced fertility of cattle | 19(26.2) | 44(57.1) | 11(16.7) |
| Reduced growth rate of cattle | 31(40.5) | 36(47.6) | 7(11.9) |
| Can cause loss of production | 47(67.9) | 22(26.2) | 5(6) |
| What do you think the effective way of disease prevention & controlling method of the following? | |||
| Vaccination | 52(73.8) | 16(19) | 6(7.1) |
| Treatment of symptomized animal | 53(75) | 16(19) | 5(6) |
| Test and slaughter or stamping out policy | 25(29.8) | 31(48.8) | 18(21.4) |
| Movement control or quarantine | 20(23.8) | 40(59.5) | 14(16.7) |
| Isolation of new purchased animal from herd | 19(22.6) | 42(61.9) | 13(15.5) |
| Decontamination of infected premises | 26(31) | 40(59.5) | 8(9.5) |
Cost of treatment of diseased animal (37.8%), death of animal due to the disease (27%), and loss of production (25.6%) are the foremost worries that respondents had felt related to respiratory disease of CBPP. Farmer’s preference ways of receiving knowledge related to animal diseases, the majority of the respondents (47.29%) preferred to receive knowledge through veterinarian/animal health workers followed by agricultural developmental agent (DA) (33.7%) whereas very few of respondents preferred to get through local cultural healers (10.8%). (63.5%) of the respondents preferred to know prevention and controlling methods followed by (14.3%) transmission methods while few farmers (4.8%) chosen diagnostic techniques (Table 11).
| Issue raised related to farmers attitude | Frequency | Percentage (%) |
|---|---|---|
| What worries you most felt if your animal diseased with respiratory disease of CBPP? | ||
| Transmission to healthy animal | 5 | 6.7 |
| Cost of treatment | 28 | 37.8 |
| Death due to disease | 20 | 27 |
| Loss of production | 19 | 25.6 |
| No worries | 2 | 2.7 |
| Which ways do you want to receive knowledge about CBPP disease? | ||
| Through district expert | 4 | 6 |
| Through DA | 25 | 33.7 |
| Veterinarian/animal health workers | 35 | 47.29 |
| Local cultural healers | 8 | 10.8 |
| Media | 2 | 2.7 |
| What worries you most felt if your animal Which part of CBPP knowledge do You want to know more? | ||
| About the causative agent of the disease | 12 | 16.2 |
| Symptoms of the disease | 1 | 1.35 |
| Transmission methods of the disease | 10 | 13.5 |
| Diagnostic methods | 4 | 5.4 |
| Prevention and controlling methods | 47 | 63.5 |
Farmer’s practices towards disease prevention and controlling techniques: In the present study result, there were very few farmers practiced good animal husbandry such as (50%) of respondents were practicing of communal grazing/watering, (50%) of respondents hadn’t used common breeding bulls with the surrounding communities, (61.9%) of respondents hadn’t purchased cattle from disease infected origin or outbreak area, (33.3%) performing isolation of new purchased animal from the herd and (38.1%) respondents were carry out restriction of freely cattle movement across the surrounding communities. Similarly, only (63.1%) of the respondents were following up vaccination of cattle. Moreover, of the total respondents (51.2%) were familiar to selling of diseased animal to neighbours or local market/butchers, (40.5%) practicing treating of diseased animal with cultural medications and only (25%) of the farmers experienced separating or isolation of diseased animal from healthy herds whereas none of the respondent exercising stamping out policy of severely diseased animals. However, (69%) of respondents were carry out treating of diseased animals in nearby of veterinary clinics. Generally, majority of the respondents were following up poor animal husbandry practices (Table 12).
| Issue raised regarding respondents’ practices associated with disease prevention and controlling methods | Response Category (N=74) | |
|---|---|---|
| Yes (%) | No (%) | |
| What are your practices you are doing during herd management to prevent contagious infectious diseases? | ||
| Avoid using common breeding bulls in the community | 37(50) | 37(50) |
| Avoiding of communal grazing & watering | 37(50) | 37(50) |
| Avoiding of cattle purchase from disease outbreak area | 32(38.1) | 42(61.9) |
| Routine manure removal or changing of kraal | 36(42.9) | 38(57.1) |
| Follow up of regular vaccination of herds | 43(63.1) | 31(36.9) |
| Isolating new purchased animal from the herd | 28(33.3) | 46(66.7) |
| Restriction of freely cattle movement | 32(38.1) | 42(61.9) |
| What are the common practices you exercised when your animal suspected or diseased with respiratory disease? | ||
| Selling to neighbours or local market/butchers | 38(51.2) | 36(48.8) |
| Presenting to veterinary clinic of area | 48(69) | 26(31) |
| Treating with cultural medications | 34(40.5) | 40(59.5) |
| Separating/isolation of diseased animal from health | 21(25) | 53(75) |
| Slaughtering for self-consumption | 28(33.3) | 46(66.7) |
Contagious bovine pleuropneumonia (CBPP) is an acute, sub-acute or chronic disease of cattle characterized by anorexia, fever and respiratory signs such as dyspnoea, polypnoea, cough, nasal discharges, fibrinous pneumonia, serofibrinous pleuritis, and oedema of the interlobular septa of the lungs (OIE, 2014). It is one of the great plagues that continue to devastate the cattle herds on which so many people are dependent and considered as the most serious infectious animal disease affecting African cattle [43,44]. Although the disease has great potential for rapid spread and causes major impact on cattle production, still nationwide epidemiological surveillance and control activities are often inadequate in most African countries including Ethiopia [45].
In all KAP study related to CBPP disease was found to be very important during the study of disease surveillance. However, the researchers did not come across on KAP study related to CBPP disease except one paper which was reported by on control of contagious bovine pleuropneumonia: knowledge, perceptions and practices in Narok district of Kenya. In our country Ethiopia many of the reported research findings related to CBPP were emphasized only on the seroprevalence of the disease. In the present study even though the existence of the disease has been confirmed through the serological test, large number of farmers had no awareness about CBPP disease in general. For instance, of the total participants 5(6%) were came across respiratory problems of cattle, on the other 55(65.5%) of the respondents have been not heard about CBPP disease and the rest of them 79(94.1%) don’t know the CBPP disease.
However, among the respondents questioned to name any infectious disease they knew, 25.7% of farmers named samba and for further confirmation, the respondents again, questioned to name any respiratory infectious disease they knew, and 11.5% were responded samba beshita, however, its scientific or English name couldn’t Identified yet. As the present study indicated the disease name was most probability might be CBPP disease, but in order to know exact local name of the disease further assessment should be made in future. Even though the name of the disease is not well-known by the communities, majority of respondents were encountered CBPP disease and undoubtedly familiarized with symptoms of CBPP. For example, Head extended coughing 61(72.6%), Laboured & painful breathing 53(63.1%), Grunting when exhaling (coughing) 56(66.7%), Frothy saliva at the mouth 49(58.3%), Swelled throat and dewlap 44(52.4%) of the respondents‟ well-informed each symptom.
As the result of KAP questionnaire indicated, majority of the participants do not aware of the possible transmission methods of contagious diseases like CBPP among cattle. However, transmission through close contact with diseased animal 55(65.5%), and coughing of infected animal 50(59.5%) are the most well-known means of disease transmission methods in the area. On the contrary, majority of respondents were aware of the impact of any respiratory diseases including CBPP. Regarding disease prevention and controlling options only vaccination 62(73.8%) and treatment of diseased animal 63(75%) are the most recognized techniques of disease prevention and controlling option in the community.
However, many of the controlling options like test and slaughter or stamping out policy of severely diseased cattle 18(21.4%), movement control or quarantine 14(16.7%), decontamination of infected premises 8(9.5%), and isolation of new purchased animal from herd 13(15.5%) were not recognized by farmers of the study area. Majority of farmers would not know what to do or would do nothing in the event of disease occurrence which agreed with the report of Kairu- Wanyoikea et al. (2014) in Narok district of Kenya. Therefore, such kind of knowledge gap among the society will be creating an ideal environment for contagious diseases like CBPP to easily distribute and infect large cattle population of the study area.
The result of this study showed regarding to farmers practice, majority of farmers were following up poor herd management practice that create favourable environment for disease multiplication and distribution across the surrounding communities of the area. Despite large number of peoples were following up poor management system of cattle production, many of farmers are voluntary or had positive attitude to apply any kind of prevention and controlling techniques of animal disease if the necessary education is afforded to them from the government. Therefore, the present study result gave hope from farmer’s point of view to create applicable disease prevention and controlling techniques through awareness creation in the communities. In a study in the UK with buffalo farmers, the authors reported that a change in practices among the farmers was necessary for the implementation of diseases prevention and controlling programs (Atnafie et al., 2015). Therefore, optimum prevention and controlling of contagious animal diseases like CBPP can be easily achieved, through changing of farmers‟ poor animal husbandry practices.
The overall animal level seroprevalence of CBPP was (16.14%, 95% CI: 11.80 -17.73) which is comparably in agreement with the findings of various researchers who reported prevalence of 12% in southern zone of Tigray region of Ethiopia [46], 14% in Niger state of north central Nigeria [47], 14.3% in Kajiado district of Kenya [48], 17.19% in Khartoum State of Sudan [49], and 17% in Turkana district of Kenya [50]. On the other hand, the finding of this study was higher than the results of [51,52] in Somali regional state (10.3%), [53] in Borena (9.4%), [54] in Bishoftu abattoir and Adama quarantine (6.85%), [55] in Borena pastoral of Oromia (0.4%), [56] in export quarantine in and around Adama (4%), [57] in Dello Mena and Sawena districts of Bale zone (6.51%), [58] in south western Kenya (9.7%), [59] in Senegal (0.43%), [60] in the Maasai ecosystem of south-western Kenya (11.21%), and [61] in north east states of Peninsular Malaysia Pertanika (8%). On the contrary, it is by far much lower than the previous reports of [62] with 31.8% in Amaro district of SNNP region, [63] with 28.5% in selected districts of western [64] with 21.05% [65] with 30.2% in agro-pastoral areas of Nigeria.
The rates of CBPP infection reported to vary from one region to another even within the region [66]. CBPP occurrence is described to cover large area of the country. Different seroprevalences of CBPP were recorded across the study districts and peasant associations such as animal level seroprevalence was (8.85%) in Kofele district while (23.4%) in Siraro (P <0.05).
Similarly, significantly higher prevalence was observed in Gayo (26.04%) PA than Wameny-Abosa (8.85%) PA. The present result of variation seroprevalence within closely related study locations (districts and PAs) was agreement with the finding of different authors such as [67] in Amaro district of SNNP region, [68] in selected districts of western [69] in Southern zone of Tigray region, [70] in Somali regional state, [71] in Turkana district of Kenya. This might indicate the presence of CBPP infection depends on certain associated risk factors like the agroecology, livestock population and movement, and different management system may be applied across the study sites such as the presence or absence of communal grazing and watering areas within locations and the probability of introduction new purchased animal from disease endemic area [72]. Therefore, the probabilities of animals to be infected with CBPP disease as well as with other diseases are various across locations even it is different within similar agro-ecological locations.
There was no significant difference of CBPP seroprevalence among the herd size which was 0.222, 95%CI: 0.602(0.267, 1.355) in medium herd size and 0.322, 95%CI: 1.445(0.697-2.994) in large herd size animal. This finding is in agreement with the work done by [73] in Amaro district of SNNP region, [74] in selected districts of western [75] in Southern zone of Tigray region of Ethiopia, [76] in agro-pastoral areas of Nigeria, and [77] in Niger state of north central Nigeria.
Age is supposed to have some association with occurrence of CBPP disease because young or calves become more resistant to infection than adult cows [78,79]. The result of this study also revealed there was statistically significant difference of CBPP disease among the age groups. Higher seroprevalence was recorded in adult animals 1.5 (1.2, 5) than in young animals. The likelihood of seropositivity of adult cattle (OR=1.5, 95% CI: 1.2, 5, P=0.017) was 1.5 times more seropositive to CBPP than young cattle. This result is in consistent with the reports of [80] in selected districts of Sidama Zone, Southern Ethiopia, [81] in Niger state of north central Nigeria, [82] in the Maasai ecosystem of south-western [83] in south western Kenya. In contrast, there are different studies that reported insignificant associations such as [84] in Amaro district of SNNP region, [85] in southern zone of Tigray region, [86] in selected districts of western [87] in agro-pastoral areas of Nigeria.
Seroprevalence of CBPP was highest in cattle with poor body condition 8.4 (1.5-10) as compared to cattle with good body conditions. This finding is in agreement with the report of [88-90]. The poor body conditioned cattle were (OR=8.4, 95% CI: 1.5-10, P=0.000) 8.4 times more likely to have the CBPP compared to the good body conditioned cattle. This could due to related to the weak protective immune response in poor body conditioned cattle than good body condition. Loss of body condition is one of the indications for the presence of the infection in the animal. Mostly CBPP chronic carrier animals became emaciated because of the clinical characteristics of the disease. Besides, animals with good body condition have relatively good immunological response to the infectious agent than animals with medium and poor body condition score [91]. This indirectly describes the impact of the disease associated with loss of productivity of cattle.
There was statistically significant between history of previous respiratory disorder problems and seroprevalence of CBPP disease (P=0.000). The prevalence was highest in respiratory disordered animals 23.9% compared to cattle that had not experienced respiratory problems 7.26%. Respiratory disordered animals (OR=2.5, 95% CI: 1.5, 4) were 2.5 times more seropositive of CBPP disease as compared to healthy animals which in line with the report [92]. Among the clinical signs of CBPP respiratory disorders like laboured painful breathing, coughing and as severity of the disease progress lung lesions was observed. CBPP is typically characterized by fibrinous pneumonia, serofibrinous pleuritis, and oedema of the interlobular septa of the lungs, frequently the symptoms of CBPP disease is associated with lung organs [93]. In this study the reason of higher seroprevalence in cattle with history of respiratory disordered animal was due to the clinical characteristics of the disease.
Lowland was significantly associated with the seroprevalence of the disease. Seroprevalence of CBPP was highest in cattle of lowland area (23.43%) as compared to cattle with highland area (8.85%). This result was in line with [94]. [95] in Southern Zone of Tigray Regions and Gedlu [96] in Somali region who reported significant difference of seroprevalence of CBPP with difference in the altitude. The lowland area of cattle was (OR= 2.6, 95% CI: 1.34- 4, P=0.001) 2.6 times more likely to have the CBPP compared to the cattle in highland area. This might be due to the fact that in lowland area there is lack of good pasture and water. Also, in lowland areas animals move long distance in search of pasture and water and they interact with other infected herds. In addition to this, in lowland areas animals are more confined to grazing area and watering point so CBPP could be easily transmitted through aerosol to susceptible animals.
The seroprevalence of contagious bovine pleuropneumonia serologically out of 384 sampled animals, 62 cattle were seropositive and this study indicated that contagious bovine pleuropneumonia was endemic in the study area. An overall 16.14% seroprevalence was recorded using c-ELISA test and of which, 8.85% recorded from Kofele while 23.4% from Siraro district. The potential risk factors like location (district), age, history of respiratory health problem, body condition score, and altitude and herd sizes were statistically significant effect on seroprevalence of the CBPP disease. Similarly, significantly higher prevalence was observed in Gayo (26.04%) PA than Wameny-Abosa (8.33%) PA. This indicates the presence of CBPP infection depends on certain geographical area, herd management system or animal husbandry, and host related potential risk factors. Even though the existence of the disease has been confirmed in the present study, there was knowledge and attitude gap among the community towards animal diseases in general and CBPP disease in particular. Besides, the majority of farmers were practicing poor animal husbandry that created favourable environment for CBPP disease multiplication and distribution across the surrounding communities. Thus, it is necessary to carry out careful herd management and control of animal movement within community and implementing regular vaccination of animals are good indicators. Therefore, based on the above conclusion the following recommendations were forwarded:
1. Further investigation in wide geographical areas of Arsi is needed.
2. The farmers should be made aware of about CBPP disease particularly the transmissions methods, and controlling techniques of the disease through veterinary extension education and possible means like media.
The government has to develop scheme and implement control measures directing at preventing further spread and lowering the prevalence of the disease in the zone through the use of therapeutic program, vaccination program and control of animal movement.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at .
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Veterinary Science & Technology received 4472 citations as per Google Scholar report