Research Article - (2023) Volume 14, Issue 1
Received: 22-Dec-2022, Manuscript No. hycr-22-84394;
Editor assigned: 24-Dec-2022, Pre QC No. P-84394;
Reviewed: 06-Jan-2023, QC No. Q-84394;
Revised: 13-Jan-2023, Manuscript No. R-84394;
Published:
23-Jan-2023
, DOI: 10.37421.2157-7587.2023.14.448
Citation: Khierallah, Amna Hassan Issa, Amel Hadj Bouazza and Daniel Montplaisir. “Comparative Study on the Efficiency of Novel Modified Chitosan-Based Electrospun Nanofibers for Removal of Fluoxetine from Wastewater Treatment.” Hydrol Current Res 14 (2023): 448.
Copyright: © 2023 Khierallah AHI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Pharmaceutical residues, along with their metabolites and conjugates, are expelled from users through urine and faeces both during and after medical therapy. The elimination of this contaminant from wastewater is therefore the subject of intense attention. In this study, the antidepressant medication fluoxetine (FLX) was removed from the solution using a new technique based on electrospun nanofibers made of N-phthalic chitosan (NPCS) and N-succinyl chitosan (NSCS) combined with PEO as a copolymer for electrospinnability. In order to achieve the best nanofiber morphology as determined by scanning electron microscopy (SEM). Adding the right chemical groups to the surface of chitosan through chemical modification allows for the elimination of pharmaceutical contaminants (FLX). Using FTIR and 1H-NMR spectroscopy, the properties of modified chitosan (NPCS) and (NSCS) were studied. Consequently, FLX was chosen as a model pollutant. Experiments on the FLX solution were used to characterize the adsorption process using a high-performance liquid chromatography (HPLC-UV DAD). The processes were well described by the pseudo-first order and pseudo-second-order kinetics, the FLX was removed by changing the pH of the experimental settings to research the impact of the solution's pH. The media's pH needs to be adjusted in order to improve pollutant removal and have a high adsorption capacity. The maximum adsorption capacities for NPCS/PEO and NSCS/PEO nanofibers, respectively, were 72.22% and 81.16%, which also showed that they were promising candidates for removing FLX from wastewater.
Modified chitosan • Chitosan phthalate • Succinyl chitosan • Fluoxetine • Electrospinning • Nanofibers • Adsorption
Water is an essential solvent for maintaining life. Water must therefore be free from contaminants and readily available in sufficient amounts. But water contamination is growing in importance worldwide [1,2]. Worldwide reports have indicated the presence of new pollutants, such as pharmaceuticals, in the aquatic environment [3,4]. One of the most studied issues throughout the years is the topic of pharmaceuticals and their presence in wastewater or the environment. Despite the years that have passed since the first mention of drug emissions polluting the aquatic environment, treatment technologies that can resolve the problem are still not in use [5]. Due to this, aquatic organisms may be affected physiologically and behaviourally by the presence of antidepressants in municipal wastewater [6,7]. The challenge is caused by the variable impurity characteristics and composition of the wastewater delivered to the treatment plant, including the low absorbability and biodegradability of cytostatic medicines. However, inadequate removal and degradation of pharmaceutical contaminants during wastewater treatment increases the likelihood that these compounds may be released into the aquatic environment [5] (Figure 1).
Antidepressants like fluoxetine (FLX) and venlafaxine (VLF), which are among the most often consumed pharmaceutical pollutants, have seen increased use as a result of the stressful lives that the majority of the world's population now leads [4]. 196 ng/L in the River St. Lawrence, Canada, was one of the highest VLF values so far discovered in an aquatic environment [8]. Additionally, FLX at values of a few ng/L have been found in surface waters [8]. The presence of FLX in the aquatic environment, even at low concentrations (few ng/L), may adversely affect aquatic organisms, such as fish, algae, and crustaceans [9]. Even at low concentrations (a few ng/L), the presence of FLX in the aquatic environment may have a negative impact on aquatic creatures like fish, algae, and crustaceans [10]. High FLX concentrations in aquatic environments, according to certain research, have led to the bioaccumulation of FLX and its demethylated metabolites in wild- caught fish, particularly in their brain, liver, and muscle tissues [10]. A common anti-depressant drug used to treat depression is FLX [11]. FLX has a certain chemical stability since it was created to elicit a particular pharmacological response, which is related to its insufficient elimination in municipal wastewater treatment plants (WWTPs) and minimal environmental degradation, as seen in Figure 1. The FLX's chemical composition [12]. Both large and tiny levels of FLX rejection are found in the urine [11] (Figure 2).
An earlier investigation found that the ability of a primary treatment method to remove and/or degrade antidepressant residues in wastewater is limited [7,13]. In the literature, other findings from the use of analytical techniques for pharmaceutical and personal care products (PPCPs) have previously been reported. The Montreal WWTPs used a physicochemical technique, which involves adding ferric chloride or alum as a coagulant and anionic polymer as a coagulant aid, to produce the results that were reported in 2006. The direct discharge of treated effluent into the St. Lawrence River has demonstrated that the amount of PPCPs removed by the physical and chemical methods used today is quite low. However, physicochemical treatment does not eliminate these molecules [7]. Adsorption is a successful tertiary way to dispose of medicines [14]. Due to their low cost and excellent adsorption efficiency, biochar materials have recently been demonstrated to be viable remedies [11]. Unquestionably, activated carbon (AC) is a strong adsorbent with a high adsorption capacity that may be employed in a variety of liquid and gas phase applications, including the treatment of wastewater [2]. However, due to the adsorbent material's potential for high operational costs for wastewater treatment systems, its use has been restricted. Therefore, it's necessary to look for less expensive options. The invention of innovative methods for FLX's adsorption and removal from wastewater is of major interest because it can be extremely hazardous to the environment and aquatic life [10]. Most medications cannot be effectively removed by conventional water treatment techniques because WWTPs are not equipped to degrade or remove these new emergent pollutants [2]. For the elimination of medicines, it is consequently vital to use a cost-effective treatment technique.
Due to its adaptability, low energy consumption, simplicity, and great efficacy in removing pollutants, the removal of pharmaceuticals by adsorption is one of the most alluring strategies for the treatment of wastewater [2]. Although recent studies have shown that commercial adsorbents like activated carbon, carbon nanotubes, and synthetic zeolites are effective and offer excellent removal rates, their high cost prevents their use in large-scale systems [15]. Therefore, there is a pressing demand for alternative, affordable, and biodegradable adsorbents. Due to their abundance in nature, low cost, strong mechanical and chemical resilience, and biodegradability, wastes originating from agriculture or forestry have recently drawn the interest of the scientific community [16]. The circular economy idea and increasingly strict environmental regulations that prevent disposal methods like landfilling and incineration are both in line with the utilization of waste materials as adsorbents [16]. Many efforts have been made to create efficient and environmentally friendly adsorbents based on affordable and natural polymeric materials in order to get beyond these limitations.
The use of CS as a potential site-specific biopolymer among natural biopolymers has received widespread acceptance [17]. According to figure 1, CS is a heteropolysaccharide made composed of amino groups, a linear polyamine, and reactive hydroxyl groups at the C2, C3, and C6 locations. In order for the structure of CS to change, these groupings are crucial. While CS with a lower degree of deacetylation is semi-crystalline, CS that is 100% deacetylated is crystalline. CS dissolves well in organic and inorganic acids but is insoluble in neutral and basic solutions. It is also soluble in mixes of water with methanol, ethanol, and acetone. The free amino and N-acetyl groups that are present in CS's structure play a role in its solubility [10]. CS is a bioproduct that is produced by treating chitin with alkali. The quality of the CS that is produced relies on the extraction conditions, such as alkali concentrations and acid-sample reaction times [18]. The amine group (-NH2) and hydroxyl (-OH) on the CS's active side give it the properties of ion exchangers [18]. Because it contains reactive amine groups, CS is easily altered. Due to their vastly different physicochemical qualities from the unmodified CS, its derivatives might offer certain advantages over it [19]. In recent years, a number of CS derivatives have been created in an effort to acquire various advantageous features over CS, which has water solubility over a wider pHrange [19,20]. CS change is required to solve this issue. CS is readily chemically altered due to the presence of amine groups (NH2) and hydroxyl (OH) from the compound. Both intra- and intermolecular hydrogen bridges can be provided by the hydroxyl and amine groups. Chitosan becomes insoluble in water as a result of the formation of a robust hydrogen network. Numerous research has been conducted to determine the effectiveness and use of CS in various applications. By introducing multiple (Figure 3) functional groups into CS structures, CS modification was done to tie CS quality [18]. Phthalic anhydride modification of CS will enhance load density, can form chitosan phthalate (CSP) compounds that are not easily dissolved by high-level substitution, and has the potential to produce ion transmitters due to the end-chain chain CO-COOH ease of ionization. Given that NPCS is a crucial intermediary that enables carefully regulated modifications to CS [7]. High affinity for organic solvents was shown by the NPCS, however it was slightly less than that of the product with additional O-phthaloyl groups [21]. The formation of N, O-phthaloylation from the reaction of CS with phthalic anhydride in N, N-dimethylformamide (DMF) required the removal of the O-phthaloyl groups through tritylation-detritylation, hydrolysis, or alcoholysis in order to produce NPCS. However, flawless functional group discrimination was made possible, and NPCS could be produced in a straightforward one-step process both selectively and quantitatively. Tritylation indicated that the resulting N-phthaloyl chitosan (NPCS) was more reactive than N, O- phthaloyl chitosan (N, O-PCS) and shows promise as a C-6 substitution precursor [21]. Anhydrous phthalates have been added to CS to diminish its crystallinity, which has increased the membrane's hydrophilic properties [18].
One of the main areas of interest in the innovative wastewater treatment techniques is nanotechnology. It is acknowledged as the upcoming technology. Researchers and scientists working in the field of nanotechnology must alter materials at the atomic and molecular levels [22]. Nanofibers can be made from a variety of substances, including natural polymers like cellulose and CS as well as synthetic polymers like polycaprolactone and polyurethane. One of its key qualities is thought to be the synthetic polymers' increased flexibility in their synthesis and modification [23]. One of the most well-known and often applied methods for creating nanofibers is electrospinning [24]. The major difficulty with this method is manufacturing nanofibers on a wide scale [25]. Numerous applications for the nanofibers produced by the electrospinning technique include filtration, tissue engineering, scaffold fabrication, wound dressings, and drug delivery [26]. Many electrospun mixtures of CS and synthetic polymers, including polyvinyl alcohol (PVA), polyethylene oxide (PEO), polyethylene terephthalate (PET), polycaprolactone (PCL), poly lactic acid (PLA), polyamide, and others, have been developed recently [27,28].
These polymers are added to improve the mechanical, biocompatible, and antibacterial qualities of CS [10]. Synthetic polymers alone or in combinations with natural and synthetic polymers can be used to create nanofibers. Due to the instability and toxicity of synthetic polymers after decomposition, which could harm cells, it is preferable to combine natural and synthetic polymers to create nanofibers with improved mechanical and biological properties [29]. Water soluble polymers poly (vinyl alcohol) (PVA) and poly (ethylene oxide) (PEO) are frequently combined to create nanofiber mats because of their superior spinnability [30]. This comparative study on the preparation and examination of chitosan succinate (CSS) and chitosan phthalate (CSP) are the main topics of this work. We changed CS to produce CSS and CSP through a substitution reaction involving succinic acid and phthalic acid. The removal of pharmaceutical pollutants from wastewater is a potential use for these novel biomaterials. The mechanism of FLX adsorption will be examined using HPLCUV- DAD and other kinetic parameters. Batch adsorption studies will be carried out under controlled settings to determine the impact of experimental variables, such as pH, on adsorption capabilities. In order to apply nanotechnology to remove FLX from wastewater, it is necessary to evaluate the sorption capacity of CSS/PEO and CSP/PEO nanofibers as well as the impact of the pH of the solution on these new CS derivatives.
Materials
Sigma-Aldrich (Reykjavik, Iceland) provided the low molecular weight chitosan (CS MW 50,000- 190,000 g/mol, 75-85% deacetylated). Polyethylene oxide (PEO) was employed as a co-spinning agent; the molecular weight average was 900,000 g/mol, Sigma Aldrich (St. Louis, MO, USA). Analytically graded substances included sodium hydroxide (NaOH), sodium chloride (NaCl) pyridine, acetone, and ethanol. Phthalic anhydride from Sigma-Aldrich (CAS 85-44-9), Succinic anhydride from Sigma-Aldrich (CAS 108-30-5) were also used. To be used as a model contaminant in the adsorption test, fluoxetine (CAS 56296-78-7) was purchased from Sigma- Aldrich (Oakville, ON, Canada). The following items were purchased from Fisher Science: methanol (HPLC grade), acetonitrile (HPLC grade), O-phosphoric acid (HPLC grade; 85 wt./wt.%), and glacial acetic acid ACS reagent (99.7%). (Ottawa, Ontario, Canada). 0.055 μS/cm conductivity (Siemens Super Transparent RO) Deionized water was used throughout all of the trials. Everything was used exactly as it had been.
Preparation N-succinyl chitosan (NSCS)
Chitosan was succinylated using the procedure described by Torres- Martínez EJ [31] and Zheng L [32], and had a low molecular weight (MW) of 50,000–190,000 g/mol and was 75–85% deacetylated. Figure 4 Depicts the creation of N-succinyl chitosan (NSCS). NSCS was produced through ringopening processes with succinic anhydride in a dimethyl sulfoxide (DMSO) framework. First, 40 mL of DMSO and 2 g of CS powder were combined thoroughly. The CS solution was then slowly added (4 g of succinic anhydride), and the reaction was allowed to run for 6 hours at 65°C. After being filtered to remove the solvent, the sample was then dissolved in ethanol at room temperature and allowed to react for an hour. At the conclusion of the process, NaOH (1 M) was added to the solution to adjust its pH to 10–12. The precipitate was then dissolved in 90 mL of distilled water, followed by the addition of 270 mL of acetone, followed by washing with ethanol and precipitation with acetone, respectively, after the mixture had been filtered. Finally, NSCS was dried at 50°C in a vacuum oven (Figure 4).
Preparation of N-phthaloyl chitosan (NPCS)
By substituting main amino groups using substituent techniques, NPCS was synthesized. The synthesis reaction of NPCS was carried out [33]. In a nutshell, CS (5.00 g, or 31 mmol glucosamine) was dissolved in 300 ml of HCl aqueous solution (0.37%) at room temperature. The polymeric solution was then vigorously stirred while phthalic anhydride solution (31.25 mmol; 4.6 g) in pyridine was added dropwise. Dropwise addition of NaOH solution was used to keep the reaction's pH at 7.0. (1.0 M). After 40 minutes, the reaction was stopped by adding 500 ml of NaCl aqueous solution (20%). After filtering, washing with acetone and diethyl ether, and drying in a vacuum oven at 50°C, the precipitate produced NPCS [17,19]. Figure 5 demonstrates the creation of N-phthalic Chitosan (NPCS) (Figure 5).
The Fourier transform infrared (FT-IR) spectrum of CS and CSP was captured using an FTIR Thermo iS10 spectrometer. The material was screened between 600 and 4000 cm-1. With chemical shifts given in ppm, the 1H-NMR spectra were generated using a 200 MHz Oxford NMR spectrometer with CS dissolved in water, NSCS dissolved in water/DH3COOH, and NPCS dissolved in DMSO-d6.
Electrospinning
It was possible to create NPCS/PEO and NSCS/PEO nanofibers by electrospinning. Figure 6 shows a schematic of the experimental equipment used to create modified electrospun nanofibers based on CS. A mixture of NSCS with PEO and NPCS with PEO were used to create the electrospinning solutions, respectively. Each polymer concentration was dissolved in acetic acid (50–90% v/v), and the results are given as w/v % (g/ml). An 8% NSCS with 5% PEO polymer solution and a 2.5% NPCS with 3% PEO polymer solution at the ratios of (6:4 NSCS) and (3:2 m/m NPCS) were prepared by dissolving the polymers in 50% acetic acid for NSCS and 90% for NPCS as the electrospinning solution at room temperature until a homogeneous viscous solution was obtained after (1 h, NSCS; 20 h PEO) The solutions were then mixed for 2 hours with constant stirring. The mixtures were then immersed in an ultrasonic bath for 15 minutes to eliminate bubbles. For the mixes to reach the necessary polymer concentrations and be prepared for electrospinning processes, they were given a final rest period of 3 hours before being utilized in the electrospinning procedure. The produced solutions were transferred to a 5 mL plastic syringe fitted with a 20-gauge needle and connected to a syringe pump that produces a continuous, slow liquid flow in order to create nanofibers. The tip of the needle and the collecting plate were placed in an electric field created by a high-voltage generator. The syringe needle's tip served as the generator's positive terminal, and a titanium frame served as the negative terminal. The syringe injection flow rate of (0.2-0.5 mL/h), a distance of (10-15 cm) between a metallic frame and the tip of the needle, and a voltage varied between (10-14 kV) at room temperature were the optimal electrospinning parameters used to create the nanofibers for NPCS/PEO and NSCS/PEO. Finally, to get rid of moisture and the last of the solvent, the electrospun NPCS/PEO and NSCS/PEO nanofibers were dried in an oven (Fisher Scientific Isotemp Oven, Thermo Scientific HERATherm Oven) at 80°C for 24 h (Figure 6).
Fiber characterization
The resulting fibre morphologies were analysed using scanning electron microscope (SEM) (JEOL JSM 5500). The obtained SEM images were further examined using the ImageJ software to measure the fiber diameters using over 100 measurements for each formulation in order to obtain an average and standard deviation (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, MD, USA, https://imagej.nih.gov/ij/,1997-2018). In comparison to any other known form of the material, the polymeric fiber exhibits many advantages when its diameter is decreased to micrometers or nanometers, including a higher surface area to volume ratio, elasticity, and improved mechanical properties (stiffness and resistance to traction). Polymer nanofibers are the best candidates for numerous crucial applications due to these characteristics [34].
Batch adsorption experiments
In each batch experiment, 25 mg of adsorbent (NSCS/PEO and NPCS/ PEO nanofiber mats) was combined with 50 mL of FLX solution and shaken at 200 rpm. Either HCl or NaOH was used, depending on the situation, to change the pH of the starting solutions. By altering the pH between 2.0 and 9.0, the impact of pH was examined. There were three duplicates of each experiment. Periodically, samples were collected, and the remaining concentration of FLX in solution was determined by HPLC analysis. (Eq.1) was used to determine the amount of FLX that was adsorbed at time t, qt (mg/g).
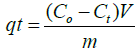 (1)
(1)
Where V (L) is the volume of the FLX solution, m (g) is the mass of adsorbents, Co (mg/L) is the initial concentration of FLX, Ct (mg/L) is the concentration of FLX in solution at time t, and (NSCS and NPCS nanofibers). Between pH 2 and pH 9, the impact of the solution pH on FLX adsorption by modification CS was examined [2]. In order to conduct kinetic studies, 50 mL of FLX aqueous solution was shaken with a chosen mass of CSS and CSP nanofiber mats (25 mg) until equilibrium was established over time intervals of 0, 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 120, and 150 min. The injection volume was fixed at 50 μl, and the column's temperature was set at 25°C. The composition of the mobile phase solution, 60:40:0.1 v% acetonitrile, water, and phosphoric acid.
To calculate adsorption capacity and comprehend the underlying adsorption mechanism, the adsorption kinetic model is frequently utilized. The pseudo-first order (PFO) and the pseudo- second order (PSO) kinetic models were used to analyses the experimental data in order to evaluate the kinetics of the adsorption of FLX among the several kinetic models that are currently in use. Pseudo-first order and pseudo-second order models were used to simulate the adsorption data for FLX at various time intervals. Non-linear pseudo-first order (Eq. 2) and non-linear pseudo-second order (Eq. 3) models were used to match experimental data reported as follows in order to clarify the adsorption mechanism involved [35].
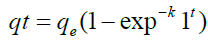 (2)
(2)
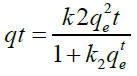 (3)
(3)
qt is the adsorption capacity of FLX at time t (mg/g); qe is the adsorption capacity at equilibrium (mg/g); k1 is the pseudo-first order adsorption rate constant (g mg−1 min−1); and k2 is the pseudo- second order adsorption rate constant (g mg−1 min−1). At pH 2, pH 7, and pH 9, the impact of solution pH on FLX adsorption by inexpensive bio-sorbents was examined. Using HCl and/ or NaOH solutions, the FLX solution's original pH was changed to the desired levels. 50 ppm of FLX solutions with starting pH values of 2, 7, and 9 were applied to the adsorbents. Samples were collected after the equilibrium period had passed, and the remaining FLX-HCl concentration was determined by HPLC analysis [16].
Shimadzu Prominence I-series high performance liquid chromatography with diode array detection was used to conduct the FLX adsorption test (HPLCUV- DAD). Using a Shimadzu with a reverse-phase column XB-C18 column, 100 Å, 150 X 3 mm, 2.6 μm particle size, and a flow rate of 0.3 mL/min [36]. The kinetic equation, Excel's solution function, nonlinear regression analysis, and MATLAB software would be used to obtain the kinetic parameters (maximum adsorption capacity and kinetic constant) for all models used in the adsorption test. With the help of the derived parameters, curves representing pseudo-first order and pseudo-second order were plotted and compared to the experimental data.
Synthesis and characterization of NPCS and NSCS polymers
Since CS has three different types of reactive groups in its repeating units and is not soluble in common organic solvents, regulated chemical modification reactions are challenging. However, in addition to the N-substitution, CS is typically somewhat O-phthaloylated after being treated with phthalic anhydride. Both phthalic and succinic anhydrides are powerful electrophiles and rapidly react with the nucleophilic amine groups of CS when present with DMF/pyridine [19,33]. In order to facilitate acylation, pyridine was added. The amino groups were likely selectively acylated because they had a stronger nucleophilic nature than the nearby hydroxyl groups (Figure 4). As shown in this study, CS and phthalic anhydride were combined to create N-phthaloyl chitosan (NPCS) (Figure 5). By substituting main amino groups using substituent methods, NPCS was synthesized. Since NPCS is soluble in several organic solvents in addition to a weak acid, it is particularly advantageous to produce the composite membranes using PEO [37]. By adding a succinyl group to the amine group of CS, N-succinyl-chitosan (NSCS) is created, improving CS's solubility in water [37,38]. Chitosan's application and basic research have been constrained by its low solubility in water and insolubility in common organic solvents. Numerous efforts have been made to produce functional derivatives by chemical changes in order to solve these issues [39]. New chitosan-functional nanofiber materials like NPCS/PEO and NSCS/PEO are being developed thanks to grafting modifications with synthetic polymers like PEO or PVA. It is likely that the ionization of the carboxylic acid moieties under alkaline circumstances, which results in the carboxylate anions, is what causes both semisynthetic polymers to have the maximum solubilities in alkaline environments. The remaining amine groups in the NPCS and NSCS chitosan derivatives have been protonated, which contributes to NPCS and NSCS's partial solubility in acidic environments. The better hydrophilic nature of the succinic moieties makes it clear that NSCS is more soluble than NPCS in aqueous media, regardless of the pH levels, as the hydrophobic aromatic rings within the phthalate moieties are expected to prevent water from penetrating them [19]. The FTIR spectra of CS, NSCS, and NPCS are displayed in Figure 6. For the CS, the -NH2 bending vibration is responsible for the absorption peak at 1650 cm−1. The –OH stretching vibration is attributed to the absorption peak at 3356 cm−1, while the -NH2 stretching vibration is attributed to the absorption peaks at 3030–3330 cm−1. Due to intramolecular and intermolecular hydrogen bonding, the infrared spectra of CS do not show an absorption peak around 3080 cm−1 [37]. The -NH2 of CS has been largely substituted by succinyl groups (-NH (CO)-CH2-CH2-COOH), turning the primary amines (-NH2) into secondary amides.
For the NSCS, two additional distinctive absorption peaks at 1648 cm−1 and 1401 cm1 correspond to the synthesis of -CO-NH-. The N-H absorption is responsible for the absorption peak at 1558 cm−1 in the NSCS spectra, according to research [40,41]. Figure 7 Shows the peaks at 3436 cm−1 for the OH group, 2887 cm−1 for the sp3 CH group, 1774 cm−1 for the imide C=O, and 1386 cm−1for the C-N group in the FT-IR spectra of the NPCS that were measured for product confirmation. Additionally, the aromatic C-C group is represented by the peak at 719 cm−1 and the C-OH group by the peak at 1061 cm−1 [37].
Further evidence for the conversion of CS to NSCS comes from 1H-NMR. The CH2CH2 moiety of the succinyl group is characterized by a triplet in the spectra of NSCS at 1.0 ppm (NHCOCH2CH2COOH). Peaks between 3.4 and 3.6 ppm are typical of CS and belong to the hydrogen in the CS backbone [38]. As a result, Figure 7 showed that these peaks may be found in the 1H of both CS and NSCS. Similar findings have been documented in the literature [41]. Figure 8 Shows the NPCS’s 1H-NMR. demonstrates important NPCSspecific signals. The aromatic region's peaks at 7.07 and 7.10 correspond to the aromatic rings from phthalate functionalities, while the peaks between 4.7 and 3.0 denote the CH of the sugar rings in NPCS [33]. Phthalate and succinate chitosan (NPCS, NSCS) were successfully produced, according to the spectra data (Figure 8).
Electrospinning and Optimization (Morphology)
The optimum diameters for electrospun NSCS/PEO nanofibers were around (18338 nm). The solvent evaporation rate during electrospinning was slow, despite the fact that the fiber diameters were large compared to the diameter of pure CS/PEO ratio 4:1 (140 ± 53 nm) as reported in our earlier work [41]. However, despite using 50% acetic acid as a solvent because it evaporates more quickly than water, we were unable to control other flaws such the generation of droplets, an unstable jet, the formation of big particles with nanofibers, and low fluoxetine adsorption capability. Fiber diameter was tracked using a single fundamental parameter, such as the concentration/ viscosity of the NSCS solution, with thicker fibers being produced as viscosity increases. The production of electrospun nanofibers is significantly influenced by the polymer solution concentration. A polymer solution's viscosity is inversely correlated with that of a solution [32]. The sample's viscosity increases with increasing nanofiber diameter. As a result, a minimal concentration is needed for fiber formation in electrospinning. We experimented with various settings to generate the continuous nanofiber with small diameters because this was the most challenging component of the electrospinning process. Additionally, when extremely little amounts of electricity are electrospun, electrospray occurs in that location. This is caused by the solution's low viscosity and high surface pressures. As shown in Figure 8 (A), the electrospinning process is greatly influenced by the concentration and mass ratio of the solution, which are both essential for producing defect-free nanofiber mats (Figure 9).
On the other side, the copolymer had the greatest impact on the electrospinning process. The best NSCS/PEO nanofiber (6:4 mass ratio%) was produced as a result of increasing the amount of PEO (Co-polymer) in the solution. Additionally, a larger voltage was required when the viscosity was low and the distance between the needle and the collector was longer. In actuality, 100% CS solutions were not evaluated. In this analysis, we tried to compare the three mass ratios (2:1, 3:2, and 6:4) when the concentration of NSCS was raised from 3% to 8%, respectively. The viscosity of the solutions was enhanced by raising the concentration of the solution in order to discover the best electrospinning settings and the greatest adsorption potential. The following settings were optimized: a flow rate of 0.01–0.5 mL/h and a configuration of 10-15 cm between the needle and the collection. The optimal voltage was determined by watching the behavior of the jet and varied from 7 to 14 kV. The three sets of parameters were 2:1 (0.01 mL/h, 8 kV, 15 cm), 3:2 (0.3 mL/h, 12 kV, 12 cm), and 6:4 (0.5 mL/h, 14 kV, 10 cm). Despite this, a pure NSCS aqueous solution cannot be electrospun because to its low viscosity. In order to increase the NSC solution's electrospinnability, PEO is frequently added to it. They are totally compatible and misciblen because to the hydrogen-bonding interactions between the hydroxyl groups of PEO and NSCS. NSC is viscous less than pure CS. Raising the voltage past a certain point led to substantially more liquid droplet projection on the collector surface as well as the destabilization of the jet since electrospinning procedures require light viscosity. Increase the solution's concentration while gradually reducing the flow rates and distance to find a solution to this issue. During an electrospinning process, a nanofiber web starts to form because the frame's corners have more surface area than the rest of the frame, which causes surface charges to accumulate. The deposition procedure will start as a "spider's web" and continue until a mesh completely encloses the interior of the frame. In addition, the use of a frame as a collector made it simpler to remove nanofibers, which made it easier for researchers to determine whether an electrospraying or electrospinning process was occurring. Even if some nanofibers were deposited, the particles would split them and prevent or reduce mat formation.
As a result, employing a farm as a collector device in an electrospinning system offers many benefits over using conventional aluminum foil. The most important advantages are reusability, a smoother mat recovery process utilizing a simple cutter, and a speedier approach to evaluate the morphology of the collected material [40,41]. We obtained nanofibers that included modest concentrations of beads and a lot of particles. The solution with an NSCS: PEO (ratio 6:4) with 8wt.%/5wt.% concentration was the optimum solution for electrospinning procedures because it was quicker, easier, and freer of particles and beads than the other methods. Additionally, it led to fluoxetine having a greater potential for adsorption. When compared to the chemical approaches used in this work, heat treatment of NSCS/PEO nanofiber membranes at temperatures between 100 and 150 o C was one of the successful methods to stabilise the membrane. Figure 8 (B). Shows NPCS/PEO electrospun nanofiber SEM pictures. NPCS 2.5% and PEO 3% mixed solutions were used to create the material. The ratio of 3:2 was discovered to be the ideal aqueous mixture (NPCS/PEO). The testing conditions were as follows: flow rate 0.3 mL/h, distance tip-collector 14 cm, voltage 12 kV, and the NPCS polymer was dissolved in 90% acetic acid. At room temperature and relative humidity levels between 30% and 40%, electrospinning was performed. As a result, our previously developed methodology [39-41]. was slightly modified in order to successfully generate nanofibers of NPCS/PEO. The ideal temperature for stabilizing nanofibers during heating was discovered to be 140 ˚C for 30 min. Without changing the surface of the fibers, membranes became stiffer under these circumstances. By electrospinning aqueous solutions after combining NPCS and PEO, nanofibers with a diameter in the range of (183 ± 38 nm) were created. It is our understanding that there is no formal literature on the production of nanofiber membranes by electrospinning NPCS PEO
General adsorption of NPCS/PEO and NSCS/PEO nanofibers
Figure 9 Shows the link between the FLX adsorption time of NSCS/PEO (mass ratio 6:4) and NPCS/PEO (mass ratio 6:4) adsorption capacities. The adsorption potential of NSCS/PEO and NPCS/PEO increases significantly in the initial phases of the reaction within 2.5 hours at 25°C. The experimental data were examined using the pseudo-first order and pseudo-second order models to ascertain the adsorption kinetics process. The pseudo-first-order rate expression with a correlation factor of R2 of 0.9926 was found to be the most accurate kinetic model for FLX sorption on NSCS/PEO nanofibers. The mechanism states that the pH of the ions at the adsorbent surface affects the rate of FLX adsorption. The rate constant (k1) is calculated to be 0.130 g/ mmol/h. FLX adsorption reaches equilibrium in 30 minutes. Figure 10 depicts the impact of time on the FLX adsorption behavior by NSCS/PEO nanofibers (Figure 10).
FLX adsorption on NPCS/PEO followed the pseudo-first-order model rather than the pseudo- second-order model. The optimum kinetic model for FLX sorption on NPCS/PEO nanofibers, on the other hand, was determined to be a pseudo-second-order rate equation (correlation factor R2=0.9926). According to the mechanism, FLX adsorption is influenced by the pH of the ions on the adsorbent surface. The computed value for K1 is 0.130 g/mmol/h. In 30 minutes, FLX adsorption achieves equilibrium. Figure 9 shows how the adsorption of FLX by NSCS/PEO nanofibers is impacted by time. At various solution pH levels, kinetic tests were conducted to ascertain the adsorption of FLX onto modified CS (NSCS) and (NPCS). the curves of kinetic energy shown in Figure 11 demonstrate that it takes roughly 2.5 hours for FLX to adsorb onto NSCS and reach equilibrium. The highest absorption, 78.5 mg/g, was attained at pH 9, among the several solution pH levels examined. The electrostatic interaction between the adsorbent and the electric charge of FLX molecules is responsible for the discrepancies between the kinetic curves produced at various pH values. For all pH values studied (pH < pKa), the molecules were primarily protonated with positive charge once the pKa of FLX hydrochloride is 9.5 [3]. The adsorbent is positively charged (+ve) at pH values between 2 and 5, which promotes electrostatic repulsion between the adsorbent and the adsorbate. The removal of FLX molecules by electrostatic attraction is enhanced at pH 8.8 because the adsorbent is negatively charged (-ve), in contrast [40] (Figure 11).
In reality, it was anticipated that FLX would have a stronger affinity for adsorption and, as a result, a higher adsorption capacity given that the Know value of its adsorption capacity into was higher NSCS than NPCS. In kinetic studies, variations in each adsorbent's behavior might be linked to the chemical makeup of the drugs. On one side of FLX, there is a highly polarized trifluoromethyl group, next to it is a highly hydrophobic benzyl group, there is an ether group in between, and on the other side of the molecule is a highly polarized secondary amine group. Given that FLX can interact more strongly with the adsorbent's surface and that the molecule's adsorption sites areb clearly defined, it may firmly bind to the surface (Table 1).
| Experimental | Pseudo first order model | Pseudo second order model | ||||
|---|---|---|---|---|---|---|
| Adsorbent | k1(min -1) | Qe(mg/g) | R2 | K2 (min -1) | Qe(mg/g) | R2 |
| NSCS/PEO | 0.01300 | 76.15 | 0.9932 | 0.00265 | 82.70 | 0.9654 |
| NPCS/PEO | 0.00526 | 73. 80 | 0.9926 | 0.00448 | 78.40 | 0.9579 |
The results displayed in Figure 10 show that the adsorption of FLX onto the NPCS biopolymer grows extremely quickly within the first 30 to 40 minutes and achieves a saturation adsorption capacity of 74% after 150 minutes. According to FTIR data and earlier research, the high adsorption capacity of (NPCS) may be caused by interactions of the –bond (associated) between the aromatic ring in NPCS and the FLX aromatic ring. However, there are strong interactions between the FLX and NPCS, such as hydrogen bonds and van der Waals interactions. As Figure 10 proposed mechanism of fluoxetine adsorption onto NPCS and NSCS (Figure 12).
The proposed mechanism of fluoxetine adsorption onto NPCS and NSCS polymers. Additionally, the FLX molecules and NPCS polymer can interact more frequently thanks to both physical and chemical adsorption into the biopolymer network. as shown by table 1. The FLX adsorption process onto the NPCS polymer can be explained by the pseudo-second-order kinetic model, according to research by (Liu, et al.). That showed a correlation (R2) of NPCS (16). This behavior is consistent with the theory that a polymer matrix with a higher proportion of polymers has an inner network of hydrogen bonds and other dipolar interactions that is denser and tighter. Since the carboxylic acid residues inside the polymer will exist mostly as the less hydrophilic unionized form, such interactions are predicted to limit water penetration through the matrix, especially under acidic circumstances. It should be noted that unaltered CS matrices totally disintegrated in the acidic medium (0.1 M HCl for 2 h), which is not surprising given that the amino groups within the CS structure are predicted to primarily exist as the very hydrophilic quaternary ammonium cations under acidic conditions. As a result, the polymer matrix can be soaked up water without resistance, leading to the polymer's final disintegration. It is clear from table 1 that chitosan succinate matrices (NSCS) absorbed more fluid than chitosan phthalate (NPCS). The higher hydrophilic nature of the succinate moieties is consistent with this behavior. It is anticipated that the hydrophobic aromatic rings of phthalate moieties will prevent water from penetrating [33].
Effect of pH solution
The pH of the solution is among the most significant variables that influence the adsorption process. The solution is influenced by pH and the electrostatic interactions between the target molecules (FLX) and the adsorbent surface (NSCS/PEO and NPCS/PEO nanofiber mats). corresponds to the pH level where the adsorbent's surface charge turns electrically neutral. The adsorbent surface gets positively charged at low pH while becoming negatively charged at high pH. (14). Lower pH results in more adsorption because all processes are more vigorous and because the functional groups on the adsorbate may become protonated, which adds to the adsorption's driving power. Additionally, molecules may be rejected by the solution in addition to being adsorbed by the forces of attraction with solids (Lyklema, 1995). Therefore, less hydrophilic substances, like FLX, may be adsorbed more readily when the ionic strength of the solution is increased by the addition of protons. According to the pKa values of carboxylic and amino functional groups, the adsorption process in the pH range under investigation may be implicated. The FLX exhibit a predominance of the species with positive charge at pH values below their pKa (9) and will reach a maximum positive overall charge at pH values 2, therefore maximizing the attraction of adsorbent [4]. As pH increases, functional groups become deprotonated (become negatively charged), which favours adsorption of the FLX (10). At pH 2, 7 and 9, the impact of solution pH on FLX adsorption by bio-sorbents (NSCS/PEO and NPCS/PEO nanofibers) was examined. The protonation/deprotonation of adsorbates and/or changes in the surface charges of adsorbents with varied pH values make the pH of a solution an especially important element in the adsorption process. The pH effect can be explained by comparing the solution pH, the pKa of the target molecule (FLX), and the adsorbent's (NSCS/PEO and NPCS/PEO nanofibers). The results shown in Figures 8 and 9 demonstrate that higher pH values, 7 and 9, were required to achieve the highest FLX uptake for all adsorbents. The electrostatic interaction between the adsorbent's surface charge and the electric charge of FLX molecules is the cause of the variations in uptake values found at various pH levels. Once FLX - HCl has a pKa of 9.8, the molecule adopts an ionized state (positive charge) at pH levels below pKa, and the ionized and nonionized forms are both present in the same proportion at pH levels equal to pKa. FLX molecules are protonated with a positive charge for each of the three pH levels considered (pH 2, 7 and 9). (pH < pKa). Because all biosorbents have positively charged surfaces at pH 2, this causes electrostatic attraction between the adsorbent and the adsorbate, which accounts for the decreased adsorption capacity at this pH level. Low-cost bio-sorbents are negatively charged at pH 7 and pH 9 (and higher), which improves the removal of positively charged FLX molecules through electrostatic attraction [16].
Several medicines and their byproducts, primarily from WWTP effluents, have been introduced in aquatic environments. The release of these contaminants into the aquatic environment has the potential to cause chronic toxicity, which can affect reproduction, as well as the emergence ofmicroorganism strains that are resistant to antibiotics. Therefore, it is vital to consider fresh approaches to lessening their impact on the environment. The current study intends to examine the efficacy of FLX adsorption capacity by a unique nanofiber material made from chitosan succinate and chitosan phthalate, which are chemical modifications of chitosan. As far as the authors are aware, there haven't been any papers or studies published that cover their use for producing modified CS nanofibers (NSCS) and (NPCS) and then using those fibers to remove FLX from aqueous solutions. The electrospinning settings as well as the solvent concentration have a significant impact on the optimal parameters electrospinning procedures for modified CS (NSCS), (NPCS), nanofibers with diameters of (150 ± 25 nm), and (183 ± 38 nm), respectively. Adsorption technology using biopolymers, like modified CS, provides significant environmental advantages, including the reduction of pollutants and the value-adding of residues that would otherwise be challenging to handle and dispose of. Alternative bio-sorbent nanofibers that can compete with commercially available adsorbents are being used; however this is still a new area of study that 600 have to be explored further. The novel biomaterials polymer has a high affinity for FLX and a 601 adsorption capacity of 80%, according to the findings of the current study. The creation of a variety 602 of contacts, including electrostatic interactions, aromatic ring interactions (-bonds), hydrophobic 603 interactions, and strong interactions like H-bonds, may be linked to the adsorption of FLX onto 604 the nanofiber's polymer (NSCS) and (NPCS). Additionally, the pseudo-first-order and pseudo- 605 second-order kinetic models can explain the adsorption process. According to the findings of the 606 current investigations, electrostatic interactions between the net surface charge of the sorbent and 607 the electric charge of the fluoxetine molecules result in a considerable influence of pH on the 608 adsorption capacity. Since the surface of the adsorbents is primarily negatively charged at these 609 pH values, which improve the electrostatic interactions with the positively charged FLX 610 molecules, the greatest absorption capacities were achieved at higher pH values, ranging between 611 (7 and 9). Real wastewater samples and the development of adsorbents that can successfully 612 remove numerous contaminants at once will be the main topics of future study. The influence of 613 numerous experimental parameters, such as temperature and beginning FLX concentration, will 614 then be determined in the adsorption method. Following that, isothermal and thermodynamic 615 adsorption data will be collected in the near future. The possibility of desorption and adsorbent 616 reuse will be discussed in the following section. This comparative investigation shows that, in 617 contrasts to pH 2, where the matrices resisted dissolving, maximal adsorption capacity release was 618 found under pH 8. More effectively than chitosan phthalate (NPCS/PEO nanofibers), chitosan 619 succinate (NSCS/PEO nanofibers) may eliminate pharmaceutical pollutants from aqueous 620 systems.
None.
None.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Hydrology: Current Research received 2843 citations as per Google Scholar report