Research - (2022) Volume 11, Issue 11
Received: 02-Nov-2022, Manuscript No. idse-21-32405;
Editor assigned: 03-Nov-2022, Pre QC No. P-32405;
Reviewed: 16-Nov-2022, QC No. Q-32405;
Revised: 17-Nov-2022, Manuscript No. R-32405;
Published:
24-Nov-2022
, DOI: 10.37421/2168-9768.2022.11.357
Citation: Tufa, Kebede Nanesa. “Review on Effects, Mechanisms and Managements of Plants Water Stress.” Irrigat Drainage Sys Eng 11 (2022): 357.
Copyright: © 2022 Tufa KN. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Water stress is a severe environmental constraint to plant productivity. Water stress induced loss in crop yield probably exceeds losses from all other causes, since both the severity and duration of the stress are critical. This Seminar describes the effects of water stress on the growth, phenology, water and nutrient relations, photosynthesis, assimilate partitioning, and respiration in plants, and the mechanism of Water stress resistance in plants on a morphological, physiological and molecular basis. Water stress reduces leaf size, stem extension and root proliferation, disturbs plant water relations and reduces water-use efficiency. Plants display a variety of physiological and biochemical responses at cellular and whole-organism levels towards prevailing drought stress, thus making it a complex phenomenon. CO2 assimilation by leaves is reduced mainly by stomatal closure, membrane damage and disturbed activity of various enzymes, especially those of CO2 fixation and adenosine triphosphate synthesis. The major mechanisms include curtailed water loss by increased diffusive resistance, enhanced water uptake with prolific and deep root systems and its efficient use, and smaller and succulent leaves to reduce the transpirational loss. Water stress effects can be managed by production of the most appropriate plant genotypes together with adjustment of agronomic practices (sowing time, plant density and soil management). Stress measurement is the quantification of the effects of environmental stresses on growth, productivity and survival of plants; Visual assessment of damages incurred Strain and measurement using different tools and protocols. Thermal sensing for plant water status, Leaf chlorophyll fluorescence, Measuring Plant Stress with an Infrared Thermometer and determination of water stress with spectral reflectance are among plant water stress measuring devices and techniques.
Water stress • CO2 assimilation • Water stress management • Measuring plant water stress
Food productivity is decreasing and affected by periodical due to detrimental effects of various biotic and abiotic stresses; therefore minimizing these losses is a major area of concern to ensure food security under changing climate. Environmental abiotic stresses, such as Water stress, extreme temperature, cold, heavy metals, or high salinity, severely impair plant growth and productivity worldwide. Water stress, being the most important environmental stress, severely impairs plant growth and development, limits plant production and the performance of crop plants, more than any other environmental factor [1]. Agricultural water stress arises from both insufficient rainfall and excess soil water during the growing season to sustain a high crop yield [2]. Plant experiences water stress either when the water supply to roots becomes difficult or when the transpiration rate becomes very high. Available water resources for successful crop production have been decreasing in recent years [2]. The growth, development, and reproduction of plants require sufficient water. Approximately, one-third of the Earth’s land area is arid and semi-arid, while periodically unexpected climatic Water stress often occur in most of the other land areas. Water scarcity can be lethal to plants and lead to enormous social problems and economic losses. The development of the modern science and technology revolution, on one hand, has largely increased our capabilities in exploring the natural resources which have dramatically improved human life; on the other hand, the continuously growing world population, together with widespread water pollution and unpredictable climatic change, further aggravates the shortage of water resources [3]. Water shortage has attracted great concern and stimulated more and more research inputs on the fundamental science of the Water stress resistance of plants and the application of the acquired knowledge for developing water saving and Water stress-resistant crops [4]. The proportion of the land surface globally, in extreme water stress (Water stress), is predicted to increase from 1 to 3% for the present day to 30% by the 2090s. The number of extreme water stress events per 100 years and mean water stress duration are likely to increase by factors of two and six, respectively, by the 2090s (Burke et al., 2006). According to the World.
Bank, water stress is the world’s most expensive disaster, destroying the economic livelihood and food source for those dependent on the agricultural sector. Much effort is being made by agricultural researchers around the globe to reduce water use by crops to address the challenges that especially affect farmers in water stress-prone environments across the developing world. Faced with scarcity of water resources, water stress is the single most critical threat to world food security. It was the catalyst of the great famines of the past. Because the world’s water supply is limiting, future food demand for rapidly increasing population pressures is likely to further aggravate the effects of water stress [5]. The severity of water stress is unpredictable as it depends on many factors such as occurrence and distribution of rainfall, evaporative demands and moisture storing capacity of soils. Investigations carried out in the past provide considerable insights into the mechanism of water stress tolerance in plants at molecular level [6]. Water stress impacts include growth, yield, membrane integrity, pigment content, osmotic adjustment water relations, and photosynthetic activity [7]. Water stress is affected by climatic, edaphic and agronomic factors. The susceptibility of plants to Water stress varies in dependence of stress degree, different accompanying stress factors, plant species, and their developmental stages [6]. Acclimation of plants to water deficit is the result of different events, which lead to adaptive changes in plant growth and physio-biochemical processes, such as changes in plant structure, growth rate, tissue osmotic potential and antioxidant defenses [5]. The responses and adaptation of crops to water deficit, and take actions to improve the Water stress resistance ability of crop plants and to ensure higher crop yields against unfavourable environmental stresses [5].
Water stress is a very complex character depending on severity, duration of the stress event, and the plant growth stage. Plant drought tolerance involves changes at whole-plant functions. For these reasons, it is necessary to determine the most suitable conditions in which to observe the type of response that is better in order to improve plant performance. Water stress induces many physiological, biochemical and molecular response on plants. Moderate to severe water stress affects various morpho-physiological traits such as chlorophyll fluorescence, water use efficiency and dry matter yield, water content and water potential membrane stability [8]. And pigment content stability drought stress progressively reduces CO2 assimilation rates owing to decrease stomatal conductance. It affects leaf size, stems extension and root proliferation, troubles plant water relations and decreases water-use efficiency. It disrupts photosynthetic pigments and reduces the gas exchange and the production of active oxygen species causes a decrease in plant growth and productivity. This review focuses on the ability and strategies of plants to respond and adapt to drought stress. Three main mechanisms reduce crop yield by soil water deficit: (I) reduced canopy absorption of photo synthetically active radiation, (ii) decreased radiation-use efficiency and (iii) reduced harvest index [9]. The reproducibility of water stress treatments is very cumbersome, which significantly impedes research on plant water stress tolerance. Although plant responses to water stress are relatively well known, plant performance under a more complex environment where multiple stresses co-occur is fragmentary. That is why the plants have to respond simultaneously to multiple stresses, e.g. water stress, excessive light and heat, which may coincide in the field [9].
Objective
• To review on Effects, Mechanisms and Managements of Plants Water Stress.
• To search on how to measure plant water stress.
Effects of water stress on plants
The effects of water stress range from morphological to molecular levels and are evident at all phenological stages of plant growth at whatever stage the water deficit takes place. An account of various water stress effects and their extent is explained below.
Crop growth and yield: The effect of water stress is impaired germination and poor stand establishment. Water stress has been reported to severely reduce germination and seedling stand. In a study on pea, water stress impaired the germination and early seedling growth of five cultivars tested Moreover, in alfalfa (Medicago sativa), germination potential, hypocotyls length, and shoot and root fresh and dry weights were reduced by polyethylene glycol-induced water deficit, while the root length was increased Growth is accomplished through cell division, cell enlargement and differentiation, and involves genetic, physiological, ecological and morphological events and their complex interactions. The quality and quantity of plant growth depend on these events, which are affected by water deficit (Figure 1). Cell growth is one of the most water-sensitive physiological processes due to the reduction in turgor pressure.
Water stress-induced yield reduction has been reported in many crop species, which depends upon the severity and duration of the stress period (Table 1). In maize, water stress reduced yield by delaying silkig, thus increasing the anthesis-to-silking interval. This trait was highly correlated with grain yield, specifically ear and kernel number per plant Following heading, Water stress had little effect on the rate of kernel filling in wheat, but its duration (time from fertilization to maturity) was shortened and dry weight reduced at maturity [10]. Water stress in soybean reduced total seed yield and the branch seed yield. In pearl millet (Pennisetum glaucum), co-mapping of the harvest index and panicle harvest index with grain yield revealed that greater Water stress tolerance was achieved by greater partitioning of dry matter from stover to grains.
| Crop | Growth stage | Yield reduction |
|---|---|---|
| Barley | Seed filling | 49-57% |
| Maize | Grain filling | 79-81% |
| Maize | Reproductive | 63-87% |
| Maize | Reproductive | 70-47% |
| Maize | Vegetative | 25-60% |
| Maize | Reproductive | 32-92% |
| Rice | Reproductive (mild stress) | 53-92% |
| Rice | Reproductive (severe stress) | 48-94% |
| Rice | Grain filling (mild stress) | 30-55% |
| Rice | Grain filling (severe stress) | 60% |
| Rice | Reproductive | 24-84% |
| Chickpea | Reproductive | 45-69% |
| Pigeonpea | Reproductive | 40-55% |
| Common beans | Reproductive | 58-87% |
| Soybean | Reproductive | 46-71% |
| Cowpea | Reproductive | 60-11% |
| Sunflower | Reproductive | 60% |
| Canola | Reproductive | 30% |
| Potato | Flowering | 13% |
Water relations: Relative water content, leaf water potential, stomatal resistance, rate of transpiration, leaf temperature and canopy temperature are important characteristics that influence plant water relations. Relative water content of wheat leaves was higher initially during leaf development and decreased as the dry matter accumulated and leaf matured Obviously, water-stressed wheat and rice plants had lower relative water content than non-stressed ones. Exposure of these plants to water stress substantially decreased the leaf water potential, relative water content and transpiration rate, with a concomitant increase in leaf temperature A conservative influence of decreased stomatal conductance in non-irrigated plants was negated by a leaf-to-air vapour pressure difference caused by the associated higher leaf temperature. Transpiration rates were similar in both treatments and the lower total water use of the non-irrigated stand resulted entirely from a smaller leaf area index Water-tolerant species maintain water-use efficiency by reducing the water loss. However, in the events where plant growth was hindered to a greater extent, water-use efficiency was also reduced significantly.
Nutrient relations: Decreasing water availability under water generally results in limited total nutrient uptake and their diminished tissue concentrations in crop plants. An important effect of water deficit is on the acquisition of nutrients by the root and their transport to shoots. Lowered absorption of the inorganic nutrients can result from interference in nutrient uptake and the unloading mechanism, and reduced transpiration flow However, plant species and genotypes of a species may vary in their response to mineral uptake under water stress. In general, moisture stress induces an increase in N, a definitive decline in P and no definitive effects on [10]. Transpiration is inhibited by water, as shown for beech but this may not necessarily affect nutrient uptake in a similar manner. Influence of water on plant nutrition may also be related to limited availability of energy for assimilation of NO3-/NH4+, PO3 4- and SO2 4-: they must be converted in energy-dependent processes before these ions can be used for growth and development of plants As nutrient and water requirements are closely related, fertilizer application is likely to increase the efficiency of crops in utilizing available water. This indicates a significant interaction between soil moisture deficits and nutrient acquisition. Studies show a positive response of crops to improved soil fertility under arid and semiarid conditions. Currently, it is evident that crop yields can be substantially improved by enhancing the plant nutrient efficiency under limited moisture supply It was shown that N and K uptake was hampered under water stress in cotton.
Photosynthesis: Plant production is mainly determined by photosynthesis, and plant photosynthesis is governed mainly by stomata for CO2/water exchange and photosynthetic activity in mesophyll cells. Water stress affects not only the light reactions, but also the assimilation efficiency of the dark reactions, thereby reducing the contents of the photosynthetic products. Plants have evolved three photosynthetic pathways including C3, C4, and Crassulacean acid metabolism (CAM) to assimilate atmospheric CO2. Generally, plants utilizing C4 and CAM photosynthetic mechanisms can better adapt to Water stress-prone climate C3 plants open their stomata during the day for CO2 absorption and fixation and close their stomata at night. This mechanism is deficient when C3 plants confront water limitation because it does not retain moisture under Water stress conditions. C4 plants have evolved a metabolic pump to concentrate CO2 in the bundle sheath cells, and perform the fixation of CO2 in mesophyll cells and the bundle sheath cells separately. This particular mechanism contributes to higher water use efficiency than that of C3 plants and provides more chance for C4 plants to survive in arid areas.
In the CAM cycle photosynthetic pathway, plants open their stomata for CO2 absorption and fixation at night, and close their stomata to reduce transpiration water loss during the day. Therefore, CAM metabolism can dramatically increase the water use efficiency and is proposed to be a plastic photosynthetic adaptation to extremely arid environments. When challenged by water stress, some plants considered as facultative CAM species are capable of switching their photosynthetic pathway from the C3 cycle to the CAM cycle mode Researchers have found that the key enzyme in the CAM metabolic pathway, phosphoenolpyruvate carboxylase, is transcriptionally regulated by water stress conditions.
Stomatal oscillations: The first response of virtually all plants to acute water deficit is the closure of their stomata to prevent the transpirational water loss. This may result in response to either a decrease in leaf turgor and/or water potential or to a low-humidity atmosphere. The debate as to whether water mainly limits photosynthesis through stomatal closure or metabolic impairment has continued for a long time. During the last decade, stomatal closure was generally accepted to be the main determinant for decreased photosynthesis under mild to moderate water When the amount of available soil water is moderately or severely limiting, the first option for plants is to close stomata. This decreases the inflow of CO2 into the leaves and spares more electrons for the formation of active oxygen species. As the rate of transpiration decreases, the amount of heat that can be dissipated increases Various experiments have shown that stomatal responses are often more closely linked to soil moisture content than to leaf water status. This suggested that stomata respond to chemical signals, e.g. abscisic acid, produced by dehydrating roots whilst leaf water status is kept constant Environmental conditions that enhance the rate of transpiration also increase the pH of leaf sap, which can promote Abscisic acid accumulation and concomitantly diminish stomatal conductance [11]. Increased cytokine in concentration in the xylem sap promotes stomatal opening directly and affects the sensitivity of stomata towards abscisic acid Comparing results from different studies is complex due to inter specific differences in the response of stomatal conductance and photosynthesis to leaf water potential and/or relative water content; the parameters most often used to assess the degree of water. It is clear that stomata close progressively as water progresses, followed by a parallel decline in net photosynthesis. However, stomatal conductance is not controlled by soil water availability alone, but by a complex interaction of intrinsic and extrinsic factors.
Photosynthetic enzymes: Very severe water stress conditions limit photosynthesis due to a decline in Rubisco activity the activity of the photosynthetic electron transport chain is finely tuned to the availability of CO2 in the chloroplast and change in photo system II under water conditions. Dehydration results in cell shrinkage, and consequently a decline in cellular volume. This makes cellular contents more viscous. Therefore, an increase in the probability of protein-protein interaction leads to their aggregation and denaturation Increased concentration of solutes, leading to increased viscosity of the cytoplasm, may become toxic and maybe deleterious to the functioning of enzymes, including those of the photosynthetic machinery.
The level of Rubisco in leaves is controlled by the rate of synthesis and degradation. However, water stress showed a rapid diminution in the abundance of Rubisco small subunit transcripts, which indicated its decreased synthesis. Rubisco activity is modulated in vivo either by reaction with CO2 and Mg2+ to carbamylate a lysine residue in the catalytic site, or by binding inhibitors within the catalytic site. Such a binding either blocks activity or the carbaxylation of the lysine residue, which is essential for activity. At night, 2 carboxyarabinitol-1- phosphate is formed in many species, which binds tightly to Rubisco, inhibiting catalytic activity. It is reported that tight-binding inhibitors can decrease Rubisco activity in the light.
A rapid decline in photosynthesis under water stress was accompanied by decreased maximum velocity of ribulose-1, 5-bisphosphate carboxylation by Rubisco, speed of ribulose-1, 5-bisphosphate regeneration, Rubisco and stromal fructose bi-phosphatase activities, and the quantum efficiency of photosystem II in higher plants Moreover, under severe water, carboxylation efficiency by Rubisco was greatly declined, and it acted more as oxygenase than carboxylase. Pyruvate or thophosphatedikinase activities were decreased 9.1 times during water stress; a much greater reduction than other enzymes, which were from 2 to 4 times, suggesting that pyruvate orthophosphate dikinase is very likely to be the limiting enzyme to photosynthesis under water stress.
Water stress resistance mechanisms: Plants respond and adapt to and survive under water stress by the induction of various morphological, biochemical and physiological responses. Water stress tolerance is defined as the ability to grow, flower and display economic yield under suboptimal water supply. Water stress affects the water relations of plants at cellular, tissue and organ levels, causing specific as well as unspecific reactions, damage and adaptation reactions. To cope with the water stress, tolerant plants initiate defines mechanisms against water deficit.
Morphological mechanisms: Plant water stress tolerance involves changes at whole-plant, tissue, physiological and molecular levels. Manifestation of a single or a combination of inherent changes determines the ability of the plant to sustain itself under limited moisture supply. An account of various morphological mechanisms operative under water conditions is given below.
Escape: Escape from water stress is attained through a shortened life cycle or growing season, allowing plants to reproduce before the environment becomes dry. Flowering time is an important trait related to water stress adaptation, where a short life cycle can lead to water stress escape Matching growth duration of plants to soil moisture availability is critical to realize high seed yield Water stress escape occurs when phenol logical development is successfully matched with periods of soil moisture availability, where the growing season is shorter and terminal water stress predominates In fieldgrown clones of Robusta coffee, leaf shedding in response to water stress occurred sequentially from older to younger leaves, suggesting that the more water-sensitive the clone, the greater the extent of leaf shedding.
Avoidance: Water stress avoidance consists of mechanisms that reduce water loss from plants, due to stomata control of transpiration, and also maintain water uptake through an extensive and prolific root system The root characters such as biomass, length, density and depth are the main water avoidance traits that contribute to final yield under terminal water environments A deep and thick root system is helpful for extracting water from considerable depths Waxy bloom on leaves helps with maintenance of high tissue water potential, and is therefore considered as a desirable trait for water tolerance.
Phenotypic flexibility: Plant growth is greatly affected by water deficit. At a morphological level, the shoot and root are the most affected and both are the key components of plant adaptation to water stress. Plants generally limit the number and area of leaves in response to water stress just to cut down the water budget at the cost of yield loss. Since roots are the only source to acquire water from soil, the root growth, its density, proliferation and size are key responses of plants to water stress. It has long been established that plants bearing small leaves are typical of xeric environments. Such plants withstand water stress very well, albeit their growth rate and biomass are relatively low. Leaf pubescence is a xeromorphic trait that helps protect the leaves from excessive heat load. Hairy leaves have reduced leaf temperatures and transpiration whilst inter- and intra-specific variation exists for the presence of this trait. Under high temperature and radiation stress, hairiness increases the light reflectance and minimizes water loss by increasing the boundary layer resistance to water vapour movement away from the leaf surface.
Physiological mechanisms: Osmotic adjustment allows the cell to decrease osmotic potential and, as a consequence, increases the gradient for water influx and maintenance of turgor. Improved tissue water status may be achieved through osmotic adjustment and/or changes in cell wall elasticity. This is essential for maintaining physiological activity for extended periods of water Wild melon plant survived water by maintaining its water content without wilting of leaves even under severe water. It has been identified that among various mechanisms, osmotic adjustment, abscisic acid and induction of dehydrins may confer tolerance against water injuries by maintaining high tissue water potential with the accumulation of solutes, the osmotic potential of the cell is lowered, which attracts water into the cell and helps with turgor maintenance. The maintenance of turgor despite a decrease in leaf water volume is consistent with other studies of species with elastic cell walls. Osmotic adjustment helps to maintain the cell water balance with the active accumulation of solutes in the cytoplasm, thereby minimizing the harmful effects of water. Osmotic adjustment is an important trait in delaying dehydrative damage in water-limited environments by continued maintenance of cell turgor and physiological processes.]
Antioxidant defense: The antioxidant defense system in the plant cell constitutes both enzymatic and non-enzymatic components. Enzymatic components include superoxide dismutase (SOD), catalase, peroxidase (PO), Ascorbate peroxidase (APO) and glutathione reductase (GAR). Nonenzymatic components contain cysteine, reduced glutathione and ascorbic acid. In environmental stress tolerance, such as water stress, high activities of antioxidant enzymes and high contents of non-enzymatic constituents are important. The reactive oxygen species (ROS) in plants are removed by a variety of antioxidant enzymes and/or lipid-soluble and water soluble scavenging molecules; the antioxidant enzymes being the most efficient mechanisms against oxidative stress. Apart from catalase, various peroxidases and per-oxiredoxins, four enzymes are involved in the ascorbate-glutathione cycle, a pathway that allows the scavenging of superoxide radicals and H2O2 Carotenes form a key part of the plant antioxidant defense system, but they are very susceptible to oxidative destruction. The β-carotene present in the chloroplasts of all green plants is exclusively bound to the core complexes of photosystem I and photosystem II. Protection against damaging effects of reactive oxygen species at this site is essential for chloroplast functioning. Here, β-carotene, in addition to functioning as an accessory pigment, acts as an effective antioxidant and plays a unique role in protecting photochemical processes and sustaining them.
Cell membrane stability: Biological membranes are the first target of many abiotic stresses. It is generally accepted that the maintenance of integrity and stability of membranes under water stress is a major component of water tolerance in plants Cell membrane stability, reciprocal to cell membrane injury, is a physiological index widely used for the evaluation of water stress tolerance. Moreover, it is a genetically related phenomenon since quantitative trait loci for this have been mapped in water-stressed rice at different growth stages showed that membrane stability of the leaf segment was the most important trait to screen the germplasm for water tolerance. Cell membrane stability declined rapidly in Kentucky bluegrass exposed to water and heat stress simultaneously in a study on maize, K nutrition improved the water stress tolerance, mainly due to improved cell membrane stability. Tolerance to water stress evaluated as increase in cell membrane stability under water deficit conditions was differentiated between cultivars and correlated well with a reduction in relative growth rate under stress. In holm oak (Quercus ilex) seedlings, hardening increased water tolerance primarily by reducing osmotic potential and stomatal regulation, improved new root growth capacity and enhanced cell membrane stability. Among treated seedlings, the greatest response occurred in seedlings subjected to moderate hardening. Variation in cell membrane stability, stomatal regulation and root growth capacity was negatively related to osmotic adjustment.
Managing water stress: Water stress effects can be managed by production of the most appropriate plant genotypes together with adjustment of agronomic practices (sowing time, plant density and soil management). This is done to ensure that sensitive crop stages occur at the time when likelihood of water is minimal. Various strategies of paramount importance to accomplish this objective may entail production of appropriate plant varieties and improvement of the existing high-yielding varieties. Efforts have been made to produce water-tolerant genotypes using the knowledge of responses of plants to water stress and mechanisms involved as elaborated above. The two most important strategies may include: (a) selecting the desired materials as in traditional breeding using molecular and biotechnological means, including production of genetically modified or transgenic plants and (b) inducing water tolerance in otherwise susceptible plants by priming and hormonal application. An account of these efforts is elaborated below.
Selection and breeding strategies: Screening under natural water stress conditions in the target environments is difficult because of the irregular and erratic water stress response. But screening under controlled stress environments and rain-out shelters is more manageable. Selection response in the target population of environments under natural stress can be considered a correlated response to selection in the managed stress environment Classical breeding is a good approach for developing water stress tolerance, which relies upon locus. Multi-location testing of progenies in environments representing a random selection of the variation in water stress in the target environment A modification to this strategy involves selection for putative water stress adaptive secondary traits either alone or as part of a selection index. Considerable efforts have been targeted at the genetic analysis of secondary traits such as root system architecture, leaf water potential, panicle water potential, osmotic adjustment and relative water content
Induction of water stress resistance: Water resistance can be induced by adopting various strategies. Of these, exogenous use of various growth regulating and other chemicals has proven worthwhile in producing water resistance at various growth stages in a number of plants. An account of these strategies is given below.
Seed priming: One of the short-term and most pragmatic approaches to overcome the water stress effects is seed priming. Seed priming is a technique by which seeds are partially hydrated to a point where germinationrelated metabolic processes begin but radicle emergence does not occur. Primed seeds usually exhibit increased germination rate, greater germination uniformity, and sometimes greater total germination percentage. This approach has been applied to overcome the water stress effects in a range of crop species. Seed priming improved performance of wheat seeds underwater stress in terms of germination and water-use efficiency of water-stressed plants by 44% compared with unprimed seeds The beneficial effects of priming included faster emergence of crop seedlings, early flowering and higher grain yield even under water stress In sunflower, osmo-priming with KNO3 and hydro-priming improved the germination and stand establishment under stress conditions.
Use of plant growth regulators: Foliar application of plant growth regulators, both natural and synthetic, has proven worthwhile for improving growth against a variety of abiotic stresses. Water stress alone inhibited increases in length and fresh weig ht of the hypocotyl, while applied levels of gibberellic acid reversed this effect. In this case, gibberellic acid partially increased the water status of the seedlings and partially sustained protein synthesis Exogenous application of gibberellic acid increased the net photosynthetic rate, stomatal conductance and transpiration rate in cotton and stimulated pollen and seed cone production in Sitka spruce (Piceasitchensis) under water stress Among other hormones, exogenous application of 1-aminocyclopropane-1-carboxylic acid also improves water tolerance by delaying senescence In another study, exogenously applied uniconazole, brassinolide and abscisic acid increased soybean yields both under well watered and water deficit conditions. Under water stress conditions, plant growth regulator treatments significantly increased water potential, and improved chlorophyll content Jasmonates, including jasmonic acid and its related compounds, are a group of naturally occurring growth regulators rather recently discovered in higher plants Jasmonates play an essential role in the signalling pathway, triggering the expression of plant defines genes in response to various stresses.
Use of osmoprotectants: Osmoprotectants are involved in signalling and regulating plant responses to multiple stresses, including reduced growth that may be part of the plant’s adaptation against stress. In plants, the common osmoprotectants are proline, trehalose, fructan, mannitol, glycine betaine and others They play adaptive roles in mediating osmotic adjustment and protecting subcellular structures in stressed plants. However, not all plants accumulate these compounds in sufficient amounts to avert adverse effects of water stress outlined three approaches to increase the concentrations of these compounds in plants grown under stress conditions to increase their stress tolerance: (1) use of traditional protocols of plant genetics and breeding to develop cultivars with natural abilities to produce high levels of these compounds under stress conditions, (2) engineering genetically modified plants capable of producing sufficient amounts of these compounds in response to environmental stresses and (3) as a short-cut method, exogenous use of these osmolytes on plants to enhance their stress tolerance ability. Exogenously applied glycinebetaine improves the growth and production of some plants under stress. In many crop plants the natural accumulation of glycine betaine is lower than sufficient to ameliorate the adverse effects of dehydration caused by various environmental stresses Exogenous application of glycine betaine has been reported to improve water tolerance in this regard Foliar-applied glycine betaine improved the growth of plants subjected to water deficit by the maintenance of leaf water status due to improved osmotic adjustment and enhanced photosynthesis, primarily due to a greater stomatal conductance and carboxylation efficiency of Rubisco Exogenous application of glycinebetaine effectively diminished the water effects in terms of greater number of achenes per capitulum in sunflower.
Agronomic practices for improving crop productivity under water stress
Promoting plant growth and development: Regulated Deficit Irrigation (RDI) places plants in a mild water stress condition for a short period of time, and once full irrigation is applied, normal plant growth and development resumes, and plants are rapidly recovered to a level similar to the fully irrigated controls. A short period of mild water deficit promotes plant development with a positive effect on plant growth. For example, in maize, the growth and development of water-stressed plants rapidly recovered to the control level only 3 days after being re-watered Timing and the extent to which RDI is applied plays a critical role in plant recovery from deficit-induced stress. In maize, deficit irrigation applied during the vegetative stage increased grain yield by 10 to 20% compared to the stress retained during the whole growth cycle Maize plants treated with mild water deficit at the seedling and stem elongation stages showed a positive “transferring effect” from early to later growth stages Compared to the fully irrigated control, the maize plants that recovered from the seedling-stage water stress were better adapted to soil water deficit occurring later in the life cycle. However, long-term severe water deficits can have a significant, negative impact on plant growth. These studies clearly show that a mild water stress can be applied at the early growth stage through RDI; this will improve the adaptability of plants to the stress through a stress-induced acclimatization process.
Stimulating root activity: There are a limited number of reports in the scientific literature that assessed the effect of RDI on plant roots. Some recent publications show that RDI generally increases root to shoot ratio. In a climate controlled environmental study, maize plants under alternate partial root-zone deficit irrigation produced 49% more rootbiomass and increased root to shoot ratio by 54%, compared to the fully irrigated control. This is a typical example where mild water stress associated with RDI has little or no effect on shoot biomass, but it promotes root growth significantly Consequently, the increased root to shoot ratio provides benefits for water and nutrient up take once full irrigation resumes. Similarly, in cotton, alternate partial root-zone irrigation stimulated the growth of secondary roots The increased secondary roots, along with increased root to shoot ratio, are beneficial for improving water absorption and enhancing soil nutrient uptake This phenomenon, commonly observed under alternate partial root zone strategy, has been validated using simulation models. In plant research, “root activity” has been used to evaluate the metabolic capacity of a root system. Root activity is usually measured using triphenyl tetrazolium chloride Roots are washed with de-ionized water and excised at a certain length from the root tips, and root tips are allowed to react with triphenyl tetrazolium chloride in phosphate buffer solution. After a certain time, sulfuric acid is added to stop the reaction. The extraction of root tips is then measured with dehydrogenases. Alternate partial root-zone irrigation has been shown to increase root activity in tomato plants by 48 to 59% compared to the conventional irrigation control Watering alternation between drying and wetting root zones with partial root-zone irrigation allows roots to experience mild water stress first, and then, re-watering provides a compensatory effect in enhancing root activity.
However, the effect of RDI on root activity may not occur in some plant species. For example, partial root-zone irrigation has no effect on root growth in oil seed rape. The tap and lateral rooting nature of oilseed allows the plants to root vertically and horizontally in response to water availability in the soil. The strong plasticity allows oil seed plants to maintain the root system that facilitates the use of rainfall and irrigation when water is available in the top soil layers and root deeper in the soil layers to absorb available water particularly under water deficit.
Maintaining or increasing plant yield: Deficit irrigation applied at the early growth stage or partial root-zone deficit irrigation has been shown to maintain or even increase yields in many field crops. In a ridge–furrow planting of cotton in arid northwest China, irrigation to alternate furrows (i.e., half the furrows were irrigated with a full amount of water while the other half were exposed to drying) increased cotton yield by 13 to 24% Mild water deficit applied in the early stage is shown to enhance the level of Water stress resistance later in the life cycle and consequently maintain or even increase plant yields Mechanisms responsible for the increased plant productivity under RDI are not well understood. However, we find the following three key factors that may have contributed to the increased plant productivity: 1. Mild deficit at the seedling stage stimulates root development and increases root to shoot ratio, so that the plants are better equipped for soil water deficit at the later stages, 2. Plants with deficit irrigation at the vegetative stage increase the remobilization of pre-an thesis carbon reserved in the vegetative tissues to the grains. Water deficit reduces the growth redundancy of stem and leaves and promotes the translocation of photosynthetic assimilates to the final products
Improving nutrient use efficiency: RDI can increase nutrient use efficiency through the promotion of nutrient recovery after a short period of water stress. For example, alternate partial root-zone irrigation to maize enhanced the ratio of N uptake in plants to the N supplied by 16% compared to fully irrigated similarly, in a maize–wheat rotation study where full irrigation and partial root-zone deficit irrigation were compared in maize, partial root-zone irrigation increased N recovery by 17% compared to full irrigation. Also, partial root zone irrigation has been shown to improve agronomic N use efficiency, apparent N recovery efficiency and N yield
Measuring plant water stress: Stress measurement is the quantification of the effects of environmental stresses on growth, productivity and survival of plants; Visual assessment of damages incurred Strain and measurement using different tools and protocols. Measuring responses of particular processes helps to determine optimum conditions. It also helps to decide which plant species, varieties or cultivars co pe a particular environmental stress better. Because water resource is becoming scarce and urban water demand is increasing, there is an urgent need to utilize water wisely for agricultural production. The key is to develop irrigation strategies for a better water use efficiency without affecting quality or quantity of yield. This requires monitoring water status of the plant frequently to properly manage irrigation. Pressure chamber has been used widely to measure leaf or stem water potential for plant water status determination and irrigation scheduling for many crops However, this conventional method is tedious and time consuming, and frequently result in an inadequate amount of sampling and is not suitable for commercial applications To address these concerns, techniques based on measuring canopy temperature have been developed. When a plant is under stress due to lack of water, it tends to close the stomata to decrease transpiration leading to an increase in leaf temperature. The energy balance of a leaf shows that this change in leaf temperature also depends on ambient conditions (i.e., relative humidity, wind speed, and ambient temperature) and radiation incident on the canopy surface. Fortunately, these parameters can be easily measured in real-time using commercially available sensors. Sensing canopy temperature using infrared thermometers or thermal cameras has shown good potential to estimate plant water status for irrigation scheduling in cotton, corn, grapevine, and pistachios Thermal imaging technique can be scaled up to large areas of crop but involves image processing techniques and can be expensive. A simple infrared thermometer with proper acquisition techniques could be used as a rapid and noncontact sensing device to evaluate plant water status.
Thermal sensing for plant water status: Response of a plant leaf to plant water status and environmental parameters can be presented by an energy balance scheme, which mainly consists of net radiation, sensible heat mostly by convection, and latent energy by evaporation across the leaf surface. For a leaf, energy generated form metabolic processes can be neglected. This model shows that leaf temperature depends on air temperature, relative humidity, solar radiation, leaf resistance, and boundary layer resistance. By utilizing proper sensors, we can study the relationship between these parameters. Air temperature, relative humidity, and solar radiation could be easily measured. Leaf temperature can be measured remotely using an infrared thermometer (IRT) by detecting infrared energy emitted. Boundary layer resistance depends mainly on the shape and size of the leaf and wind speed. Wind passing through the leaves of many plants can be approximated as laminar flow over flat plates Leaf resistance (rL) can typically be measured using leaf Porometer.) found good correlation between leaf and stem water potential and leaf resistance in almonds.
Determination of water stress with spectral reflectance: The use of remote sensing for irrigation practices, water resource management, and disease and insect management has been largely investigated The spectral characteristics of healthy vegetative surfaces are distinctive with low reflectance in blue, high in green, very low in red and very high in the near infrared (NIR). The overall reflectance of water in the visible region (400–700 nm) is relatively low and in the NIR (700–900 nm) it is practically zero Extensive research has been conducted to study pigment concentration of plants using spectral reflectance under various environmental conditions and stresses A large number of vegetation and water indices have been developed to measure plant vigour and other biophysical parameters using remotely sensed data. The leaf water content index measures leaf relative water content directly and can be used to determine when certain plant species with different leaf morphologies are water stressed. Reported that non-invasive monitoring using hyper-spectral vegetation indices could improve current traditional methods for estimating water status of individual vines. Spectral vegetation indices were designed to evaluate vegetation condition, foliage, cover, phenology and processes in addition to be used for land cover classification, climate and land use detection, Water stress monitoring and habitat loss discussed that spectral vegetation indices are mathematical expressions involving reflectance values from different part of the electromagnetic spectrum, aimed to optimize information and normalize measurements made across varied environmental conditions. Varied environmental conditions include differences in plant species, solar angle, shadowing, illumination, canopy coverage, soil background, atmospheric condition and viewing geometry of the device over space and time. Since there are many water absorption and reflection features in the red and NIR parts of the electromagnetic spectrum, analysis of spectral reflectance has resulted in several useful water indices. A few water indices developed to study crop stress include the water band index (WBI), shortwave infrared water stress index (SIWSI) developed by Fensholt and Sandholt (2003), and normalized difference water index (NDWI). In addition to vegetation indices, many statistical and mathematical models such as principle component analysis random forest, support vector machine, artificial neural network, and other classification procedures have been developed to extract optimal information from remotely sensed data.
Measuring plant stress with an infrared thermometer: Infrared thermometers are rapid, reliable instruments for measuring foliage temperature. These instruments are relatively simple to use with only a few considerations, e.g., field of view, target dimensions, and calibration. The foliage temperature can be incorporated into crop water stress indices that have been related to soil water availability and leaf water potential. The number of samples that need to be collected is relatively small. The infrared thermometer provides a technique for the remote detection of stress in all types of plants. Plant stress measurements with the hand-held infrared thermometer (IRT) have become increasingly popular in the last 10 years following the introduction of the portable, battery-powered IRT. Digital displays of foliage temperature allowed for quick and easy measurements. Possible sources of error and potential uses of the IRT to measure water stress will be presented. It is assumed that the reduction of soil water will result in stomata closure and cause an increase in foliage temperature.
Leaf chlorophyll fluorescence: The photosynthetic apparatus has been recognized as being a good indicator of stress and stress adaptation of a plant and is associated with the measurement of chlorophyll. Also, because changes in chlorophyll fluorescence may occur before any physical signs of tissue or chlorophyll deterioration are manifested in the plant, stress can be detected before the onset of physical damage Chlorophyll fluorescence measurements can be described using the typical phases of a temporary fluorescence signal or transient. During a typical fluorescence transient, the fluorescence rises rapidly from a ground state, O (or Fo) initial or minimal fluorescence, when all electron acceptors are fully oxidised, or open, to a maximum level, P (or F m), when all electron acceptors are highly reduced, or closed and are unable to accept and transfer electrons Various parameters representing subsequent phases in a typical fluorescence transient can yield information on how stress affects the functioning of the photosynthetic system. Photochemical efficiency is a common parameter used to assess the effect of environmental stresses on the photosynthetic mechanism. The photochemical efficiency of Photo-system II (PSII) is estimated by Fv/Fm, which is the ratio of variable fluorescence (Fv) to maximum fluorescence (Fm). Most healthy plants exhibit F v/Fm values of around 0.8iIn most studies on the applications of chlorophyll fluorescence, the Fv/Fm ratio is used as an indicator of water stress
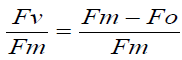 @
@
In these studies, it has been well documented that at the chloroplast level, the function of the thylakoid membrane is sensitive to environmental stress Studies which have focused on deep-rooted exotic tree species have suggested that a decrease in Fv/Fm is due to Water stress-induced injury to the thylakoid structures affecting photosynthetic electron transport Rolando and Little (2003) also showed a decrease in Fv/Fm of water-stressed Eucalyptus grand is seedlings, resulting from a rise in Fo and a decrease in Fm. Since this ratio is a reflection of the maximum yield of primary photochemistry, Fv/Fm is also used as an indicator of tree or seedling vigour. Water stress leads to several other changes in the photosynthetic apparatus of plants. Low water potential has been observed to cause a decrease in the quantum yield of O2 evolution in chloroplasts and leaves from sunflower plants; a decrease in the ability of the coupling factor isolated from spinach leaves to bind fluorescent nucleotides; and a decrease in the ratio of the maximum to the minimum fluorescence in the red algae Porphyrs sanjuanesis. In all cases examined, the ratio decreased as the water potential decreased. Because of these results, concluded that water stress inhibited electron flow of PSII in the 3 species examined, and that this ratio serves as a qualitative indicator of leaf water potential. The use of chlorophyll fluorescence ratios as an index of plant water stress has gained increasing acceptance in recent years, and is commonly measured using hand-held relatively low-cost portable instruments which are simple, rapid and non-destructive. With the development of an internal saturating light source in portable field fluorescence meters, chlorophyll fluorescence measurements can now be undertaken at any time of the day, from shaded or sunlit samples. Chlorophyll fluorescence measurements can be used in conjunction with other techniques as a relatively quick initial screening method for assessing plant stress within a localized area.
Leaf-water content: Relative leaf water content is an indirect and gross estimate of the changes in the water content in leaves (Canny and Huang, 2006). Most water in leaves resides in mesophyll cells. Volumetric changes in these cells occur as the balance shifts between the rate of evaporation from leaves and the rate of water supply to the leaves. Volumetric changes in the leaves of plants affect many internal plant conditions such as tension in the cell walls, exchange of water and carbon dioxide across cell membranes, osmotic pressure of vacuole contents, cell and tissue turgor, cell-to-cell contact and transport of water. Measurements of the relative water content of leaf tissue are commonly used to assess the water status of plants.
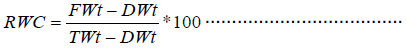 @
@
Where: RWC – Relative Water content, FWt – Fresh weight, DWt – dry weight, TWt- turgid weight. The validity of relative water content measurement depends on the precision of the three weight determinations; a reliable estimate of turgid weight being the most critical. A typical water absorption curve for a leaf shows a high initial rate of absorption, followed by a prolonged period of slow absorption. The amount of water initially absorbed has been commonly interpreted as being the amount of water needed to compensate for the water deficit of the plant tissue. Further water absorption is driven by cell expansion, so that mass changes occurring during this phase are not used in the estimation of the relative water content of the sample. Therefore, an accurate measurement of turgid weight should be determined at the end of the first initial phase of water absorption.
Water deficit reduces plant growth and development, leading to the production of smaller organs, and hampered flower production and grain filling. Timing, duration, severity and speed of development undoubtedly have pivotal roles in determining how a plant responds to water deficit. Following drought, stomata close progressively with a parallel decline in net photosynthesis and water use efficiency. Stomatal conductance is not controlled by soil water availability alone, but by a complex interaction of intrinsic and extrinsic factors. Depending upon the availability of moisture, activities of the enzymes of carbon assimilation and those involved in adenosine triphosphate synthesis are decreased and sometimes inhibited. One of the major factors responsible for impaired plant growth and productivity under drought stress is the production of reactive oxygen species in organelles including chloroplasts, mitochondria and peroxisomes. The reactive oxygen species target the peroxidation of cellular membrane lipids and degradation of enzyme proteins and nucleic acids.
Being very complex, the drought tolerance mechanism involves a number of physiological and biochemical processes at cell, tissue, organ and whole-plant levels, when activated at different stages of plant development. Examples of these mechanisms are reduction in water loss by increasing stomatal resistance, increased water uptake by developing large and deep root systems, accumulation of osmolytes and osmoprotectant synthesis. Drought stress effects can be managed by production of most appropriate plant genotypes, seed priming, plant growth regulators, and use of osmoprotectants, silicon and some other strategies. Although physiological mechanisms of drought tolerance are relatively well understood, further studies are essential to determine the physiological basis of assimilate partitioning from source to sink, plant phenotypic flexibility which leads to drought tolerance, and factors that modulate plant drought-stress response. However, an understanding of root responses to drought stress, most likely involving root-shoot signaling, is a preferred area of research. Investigations that seek to improve crop performance by increasing osmotic adjustment need to focus on meristematic regions of roots. For effective application and commercial use of exogenous glycinebetaine, proline and other compatible solutes as inducers of drought tolerance, their mechanisms of action, the most optimal concentrations, and appropriate plant developmental stages must be carefully determined. The role of H2O2 as a signaling molecule as well as the identification of regulatory components in the pathway that leads to plant responses to drought stress are fundamental clues for future research. Applications of genomics, proteomics and trascriptomic approaches to a better understanding of the molecular basis of plant drought tolerance and improved water-use efficiency under drought are also imperative. Mutants or transgenic plants exhibiting differential capabilities for reactive oxygen species formation and elimination could be useful to elucidate this fundamental point. Molecular knowledge of response and tolerance mechanisms is likely to pave the way for engineering plants that can withstand and give satisfactory economic yield under drought stress.
Measuring responses of particular processes helps to determine optimum conditions. It also helps to decide which plant species, varieties or cultivars cope up a particular environmental stress better. Because water resource is becoming scarce and urban water demand is increasing, there is an urgent need to utilize water wisely for agricultural production. The key is to develop irrigation strategies for a better water use efficiency without affecting quality or quantity of yield. Water deficit affects the development, growth and yield in plant crop, but the tolerance crops to this stress varies remarkably. Changes in morphological, physiological, biochemical and molecular aspects are generally noted in response to drought stress. Understanding these responses to drought is important for screening tolerance of genotypes to water-limited conditions.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Irrigation & Drainage Systems Engineering received 835 citations as per Google Scholar report