Case Series - (2022) Volume 13, Issue 8
Received: 29-Oct-2019, Manuscript No. JVST-19-3996;
Editor assigned: 04-Nov-2019, Pre QC No. JVST-19-3996 (PQ);
Reviewed: 22-Nov-2019, QC No. JVST-19-3996;
Revised: 29-Jul-2022, Manuscript No. JVST-19-3996 (R);
Published:
26-Aug-2022
, DOI: 10.37421/2155-6113.2022.13.125
Citation: Wondimu, Anteneh. “Prevalence and Seasonal
Dynamics of Gastrointestinal Nematode of Goats in Haramaya, Ethiopia .” J
Vet Sci Technol 7 (2021) : 125.
Copyright: © 2022 Wondimu A, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits
unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Gastrointestinal nematodes are one of the main constraints to goat production worldwide. A cross-sectional study longitudinal study from were conducted in Haramaya, Ethiopia to determining the prevalence and seasonal dynamics of gastrointestinal nematodes of goats. A total of 384 fecal samples were randomly collected and analyzed using standard parasitological methods of floatation and ova culture. The overall prevalence of gastrointestinal nematodes was 73.7% (283/384). The result showed higher percentage prevalence in young (76.2%) than adult’s age group (73.0%). The difference in gastrointestinal nematode infection between female (79.6%) and male goats (59.09%) was statistically significant (p=0.001). The Egg Per Gram (EPG) count result showed 69.6%, 7.4%, 23.0% of the goats were lightly, moderately and heavily infected with gastrointestinal nematodes respectively. The overall genera of nematode identified and counted from coproculture in the study areas were trichostrongylus spp (54.6%), teladorsagia (17.6%), haemonchus spp (14.4%), muellerius capillaris (7.6%), oesophagostomum spp. (1.7%), nematodirus spp (1.7%), strongyloides papillosus and cooperia (0.9%), chabertia (0.5%) and trichuris (0.1%). Based on the monthly mean EPG count recorded during the short and long rain seasons with peaks occurring in may and september of the year. To mitigate the burden of gastrointestinal nematode parasites: Good management practices considering breed, sex, age, pasture rotation, housing of animals during peak time and optimizing anthemintic usage through deworming of goats during season where parasitic load is peak in host animal is very crucial.
Gastrointestinal Nematodes • Goats • Prevalence • Haemonchus
Small ruminants are an important financial source of the country as they provide more than 30% of local meat consumption and generate cash income from the export of meat, live animals and skins [1]. Goats are an essential livestock species of animal especially in tropical and subtropical regions in small scale farming and rural economy through generating employment and supplementing house hold income [2]. Despite the large number of sheep (24.2 million) and goats (22.6 million) population in Ethiopia, the economic benefits remain marginal due to prevailing diseases, poor nutrition, poor production systems, reproductive inefficiency, and management constraints [3]. Among the factors diseases have a major impact on morbidity and mortality, with annual losses as high as 30%-50% of the total value of livestock products of Ethiopia [4].
Gastro Intestinal (GI) nematodes are worldwide problem associated with reduction in the production of livestock in many countries; their impact is greater particularly in sub-saharan africa due to the availability of a wide range of agro-ecological factors suitable for diversified host and parasite species [5]. According to the report of the epidemiology of GI parasites in livestock varied depending on the local climatic condition, such as humidity, temperature, rainfall, vegetation and management practices. Helminthes parasites of ruminants are ubiquitous in all of the agroclimatic zone of Ethiopia and responsible for the death of one third of calve, lamb and kids, and considerable losses of parts of carcasses [6]. Mixed infections with different GI nematodes are common in different part of Ethiopia, the most important genera affecting small ruminants includes: Haemonchus, Trichostrongylus, Oesophagostomum, Bunostomum, Strongyloides, Cooperia, Nematodirus and Trichuris [7].
Reports from different scholars showed small ruminants harbor one or more genera of GI nematodes with prevalence rate of 49.2%-78.7% in different parts of Ethiopia. Previous study conducted ten years before on prevalence and intensity of GI nematodes in the same study area stated the prevalence of nematodes in sheep and goats, with Haemonchus contortus being the most prevalent (65%-80%), followed by trichostrongylus. Knowing the current situation of GI nematodes and seasonal dynamics in the area could be the basis for all possible actions including its control and prevention. Therefore, the present study is, aimed at determining the prevalence and seasonal dynamics of goats gastrointestinal nematode with associated risk factors in Haramaya Ethiopia [8-15].
Study area
The study was conducted Haramaya university goat farm and nearby kebels of Haramaya, Eastern Hararghae, Ethiopia. The area is approximately 14 km from west of Harar and 510 km east from Addis Ababa. The estimated animal population in the area is about 63,723. Topographically, it is situated at altitude of 1600 m to 2100 m above sea level with the mean annual temperature and relative humidity of 18°C and 65%, respectively. Geographically it is located 041° 59' 58" n latitude and 09° 24' 10"s longitudes. There are four seasons; a short rain season a short dry season, a long wet season and a long dry season [16]. The Haramaya area receives an average annual rain fall of approximately 900 mm, with a bimodal distribution pattern picking.
Study population
In this study a total of 384 goats were used for coprological examination. The study animals included from small holder, where farmers maintain one to three goats and semiintensively reared goats [17]. Around 246 goats of Hararghae breeds from nearby Haramaya and 138 goats of Somale, Boar, Angello, Abergele and Hararghae breeds from Haramaya University goat farms were used to determine prevalence, associated risk factors and the seasonal dynamics of GI nematodes [18].
Study design
A cross-sectional study used to determine the prevalence and associated factors and longitudinal to see the seasonal dynamics of GI nematodes in the study area [19]. During the study period fecal samples were randomly collected from different breeds of goats in the study area. The considered explanatory variables were: Age, sex, breed, and genera of parasites.
Sample size determination
Goats maintained in traditional husbandry and semiintensively system was used to assess epidemiology and seasonal dynamics of GI nematodes. The sample size is calculated with a 95% significant level and 5% marginal error. The sample size is determined by the formula of Thrusfield [20-23].
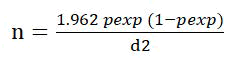
n= required sample size
Pexp=expected prevalence
d= required precision
The expected prevalence of GI nematodes of small ruminants is 80% in eastern Ethiopia. Based on the above formula the total sample size is 246. To increase the precision by 0.1% 384 goats included in the study.
Coproscopic examination and EPG determination
Approximately 10 g-15 g faecal samples were collected directly from rectum of each goats and where possible for freshly voided faeces. The faecal sample was put in a sterile bag coded with the date, origin, breed, age and sex of the animal and transported to Haramaya University Veterinary Parasitology Laboratory and immediate further processing is done. At one monthly intervals faecal samples was collected from the goats to determine the concentrations of the parasite eggs (EPG). Faecal samples are examined for helminth eggs using the modified McMaster technique with saturated sodium chloride solution as the floating medium. 3 g of faeces are mixed in 42 ml of saturated salt solution, and the number of nematode Eggs Per Gram of faces (EPG) is obtained by multiplying the number of nematode eggs counted in two squares of the Mc Master slide by a dilution factor of 50. The degree of severity was categorized based on previously described methods. In general, the category of intensity of infection was made based on faecal EPG counts as light (50 epg -800 epg), moderate (800 epg-1200 epg) and heavily (>1200 epg). Eggs of the different nematode parasites were identified on the basis of morphological appearance and size of eggs as described [24].
Ova culture and larval identification
After determination of EPG composed faecal samples was cultured for larvae identification [25]. Faecal sample containing parasitic eggs were placed in small sized plastic and placed at room temperature (approximately 22°C-25°C) for 14 days. The sample was regularly checked and again little water if it was to the dried one. Then at the end of 14 days the sample was subjected to the modified Baermann technique. Centrifuge tubes are filled to two thirds with medium and larvae is spin at 1500 rpm for 5 minutes in an electrically powered centrifuge to concentrate the larvae to the bottom. The larvae are recovered and pooled together after decantation of the supernatant fluid. Active larval movement is observed when viewed under the microscope with × 10 objective. A drop of the recovered larvae is stained with lugol’s iodine, viewed and counted, identified to genera under light microscope at × 40 objectives [26].
Data analysis
The data obtained were recorded in Microsoft excel spread sheet and analyzed by SPSS version 20. The presence of associations between associated factors and parasitism were compared using Chi-square (x2 ) test and the level of significance was set at p<0.05. The strength of association between variables was determined using odds Ratio [27].
Out of 384 examined goats 283 were found to be infected with 73.7% overall prevalence of GI nematodes. The degree of infection based on EPG count showed, the highest percentage of goats were lightly infected (69.6%) followed by heavy (23%) and moderate (7.4%) infection The current finding was comparable with 70.7% to 71.45% prevalence report of different scholars in Ethiopia [28]. In contrary, the result is lower than the reports of somewhere in Ethiopia. But the result of this study is higher than the report with prevalence rate of 49.2%, in Dembia District, Ethiopia. The difference in the prevalence of GI nematodes in different area could be due to a variation in humidity and temperature which commonly supports parasitic growth and development (Table 1).
| Intensity of infection | Minimum-Maximum | No. sample | Proportion |
|---|---|---|---|
| Light | 50-800 | 197 | 0.696 |
| Moderate | 800-1200 | 21 | 0.074 |
| Heavy | 1200-37400 | 65 | 0.23 |
Among all breeds of goats examined, Abergele breed showed the highest GI nematode prevalence (90.9%), followed by Angelo (88.0%), Somale (74.6%) and Boer breed (58.8%).
The result indicated there is significant difference between infection of Hararghae and Angello breeds of goats (p-value 0.018, OR=0.143). Breed may be one factor that affect the degree of nematodes infection (Table 2).
| No. examined Variables | No. (Positive, Negative) | 95% C.I. for OR | Odds Ratio | 95% C.I. for OR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Breed | Prevalence | Chi2 | P-value | Lower | Upper | ||||
| Somale | 57 | 42, 15 | 0.737 | 2.922 | 0.087 | 0.275 | 0.062 | 1.209 | |
| Boar | 34 | 20, 14 | 0.588 | 2.705 | 0.1 | 0.272 | 0.058 | 1.283 | |
| Angello | 25 | 22, 3 | 0.88 | 5.64 | 0.018 | 0.143 | 0.029 | 0.712 | |
| Abergele | 22 | 20, 2 | 0.909 | 0.49 | 0.484 | 0.525 | 0.086 | 3.19 | |
| The reference category used: Hararghae breed | |||||||||
| Sex | Male | 110 | 65, 45 | 0.5909 | 16.9 | 0.001 | 2.7 | 1.6 | 4.4 |
| Female | 274 | 218, 56 | 0.796 | - | - | - | - | - | |
| Total | 384 | ||||||||
The result showed presence between of statistical association different sex groups in the prevalence of GI nematode, higher prevalence in female (79.6%) than in male (59.09%) goats (p-value 0.001, OR=2.7). This was consistence with the report in Northern Ethiopia and Kaffa and Bench Maji Zones, Ethiopia who reported a higher prevalence of helminth infection in females [28]. Most of the earlier researchers in different part of the world have observed higher rates of nematode infection or worm burden in female animals compared with the males. The higher prevalence of nematode parasites in female goats may be because of lowered resistance of female animals due to their reproductive events and insufficient or unbalanced diet during higher needs [29].
According to this study, age group prevalence of nematode The overall nematode genera identified and counted from coproculture in young (76.2%) than adult goats (73.0%) but not significant statistically (p-value>0.05). This finding was in agreement with the reports of scholars in different parts of Ethiopia, who reported higher prevalence of GI nematods in young than adult goats [30]. The higher prevalence in young animals may be due to exposure to contaminated environment, overstocking and lack of immunity. As the age increase the prevalence of GI nematodes in small ruminant’s decreases due to well-developed immunity against GI nematodes (Figure 1).
The overall nematode genera identified and counted from coproculture in the study areas were Trichostrongylus spp (54.6%), Teladorsagia spp (17.6%), Haemonchus spp (14.4%), Muellerius capillaris (7.6%), Oesophagostomum spp. (1.7%), Nematodirus spp. (1.7%), Strongyloides papillosus and Cooperia (0.9%), Chabertia (0.5%) and Trichuris (0.1%). The most dominate nematodes were Trichostrongylus spp, Teladorsagia spp, and Haemonchus spp. These nematode parasites found in this study also been reported by different scholars in the country who documented a higher prevalence of Trichostrongylus spp followed by Haemonchus spp. However, it is contrary to the reports of other authors who reported Haemonchus spp being the most prevalent nematode parasite of goats in the study area [31-33]. The difference in the prevalence of different nematode species from previous reports could be due to sample analyzed during dry season of the year where Haemonchus undergo hypobiosis.
During the study period monthly EPG count showed seasonal variations generally in accordance with the wet and dry seasons. The EPG increased with the onset of the rains to reach peak levels towards the end of the long rain season and also peak at the end of short rain season. Subsequently, the EPG decreased during the long dry period similar environmental factors reported by several scholars. For example there is existed direct relationship between moisture and prevalence of parasitosis while desiccation suppress the development and growth of parasites thereby reducing the infection rate. The prevalence of GI parasites, the genera of helminth parasites involved, species and the severity of infection also vary considerably depending on local environmental conditions, such as humidity, temperature, rainfall, vegetation and management practices.
The present study showed that GI nematode parasitosis was prevalent disease in the area affecting the wellbeing of goats. Around, 73.7% of goats harbor one or more genera of GI nematodes. Young and females are relatively heavily infected with nematode parasites. Trichostrongylus spp, Teladorsagia spp, and Haemonchus spp were the dominant nematodes identified from larvae culture. The nematode burden increased with the onset of the rains to reach peak levels towards the end of the long rain season and decreased during the long dry period. To mitigate the burden of GI nematode parasites: Good management practices considering breed, sex, age, pasture rotation, housing of animals during peak time and optimizing anthelmintic usage through deworming of goats during season parasitic load is peak in host animal.
The authors gratefully acknowledge Haramaya University for financial support and Jenber Abera for excellent technical assistance during laboratory activity.
The authors declare that they have no competing interests.
Veterinary Science & Technology received 4472 citations as per Google Scholar report