Research Article - (2023) Volume 10, Issue 3
Received: 02-May-2023, Manuscript No. JREAC-23-98872;
Editor assigned: 05-May-2023, Pre QC No. JREAC-23-98872(PQ);
Reviewed: 19-May-2023, QC No. JREAC-23-98872;
Revised: 22-May-2023, Manuscript No. JREAC-23-98872(R);
Published:
19-Jun-2023
, DOI: 10.37421/2380-2391.2023.10.421
Citation: Kahlid, Maryam. "Optimal Generation
of Renewable Energy from Non-Edible Biomass via Pyrolysis." J
Environ Anal Chem 10 (2023): 421.
Copyright: © 2023 Kahlid M. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Pollution in the environment and reduced fossil fuels supply urges to use non-edible biomass as renewable source of energy. Plant waste is observed more environmentally friendly than fossils fuels as renewable, sustainable and efficient biomass resource. Pyrolysis is one of the thermo chemical processes most effective for waste into energy product conversion and is an evolving technology. In present research, the pyrolysis of non-edible biomass (peach seeds and seed kernels) was performed to confirm its potential for renewable energy production. The maximum yield of Peach seed bio oil was 51% obtained at 550°C temperature while the maximum yield of Peach seed kernel bio oil was 41.5% obtained at 450°C temperature, 1mm of particle size, 90 min of reaction time and 200 cm3/min of nitrogen rate of flow. Bio oils obtained after pyrolysis process were analyzed for physical and chemical properties. Chemical properties determined by FTIR showed the presence of polymeric OH, alkanes, alcohols, aldehydes, ketones, carboxylic acids, alkynes, esters, ethers and aromatic compounds in peach seed and seed kernel bio oils.
Renewable energy • Pyrolysis • Non-edible biomass • Peach seeds
Environmental concerns and future energy safety are the main incentives of increased use of biomass worldwide, particularly in developing countries such as Pakistan. It is necessary to know about the current energy system in different sectors, in order to allow effective use of native resources of biomass. In the context of current energy scenarios, renewable energy should be explored broadly in order to renew energy resources and keep sustainable development safe. The key factors contributing to the search for alternative energy sources are declining availability of fossil fuels and environmental pollution. Biomass is cheap, environmentally friendly and abundantly available. Overall, biomass is the fourth largest energy source currently and provides approximately 15 percent of the energy used [1].
Biofuels, including solid, liquid and gaseous materials could be obtained from different biomasses, which can be divided by their origin into first, second, third and fourth generations. Firstgeneration biofuels are those obtained from food crops. Biofuels of second-generation are derived from feedstocks of lignocellulosic nature and waste materials. Biofuels of the third-generation are obtained from algal biomass and represent the most promising renewable resource. Fourth-generation bio-fuels are obtained from genetically modified micro-organisms such as cyanobacteria, microalgae, yeast and fungi [2].
The biomass feedstock is capable, environmentally friendly and alternative source of renewable energy in today's energy scenarios. Biomass as renewable energy source could play an important role in meeting the demand of energy sectors globally. It is one of the largest sources of energy accounting for 14% of the 18% renewable energy source. Figure 1 shows the biomass distribution as a source of renewable energy all across the world [3].
One of the possible sources of renewable energy is plant waste materials. The change of plant waste into an acceptable form of energy, generally as fuel or electricity could be accomplished using different number of paths. Biomass is a sink of carbon and contains far less Sox and Nox than coal or synthetic oil because of the much lower level of nitrogen and sulphur in biomass. Biomass use as a source of renewable energy is very fascinating because it provides a considerable development towards being environment friendly. Actually, the use of biomass processing technology offers a solution to the problem of pollution and creates new employment in the area of revolutionary advances in agricultural waste usage. The conversion of biomass offers further benefits by reducing the volume which enables its transport and storage easy and saves it from deprivation if stored for a long time. Biomass can be transformed into bio-oil through various biological, physical and thermal processes. Amongst the processes of biomass conversion to energy, pyrolysis has concerned greater attention in biofuel production due to its benefits in storing, transporting and flexibility in various technologies [4].
The word pyrolysis is derived from Greek words, “pyro” meaning fire and “lysis” meaning breakdown into fundamental parts. Pyrolysis is actually thermal conversion in which feedstock’s of biomass is changed into bio-oil at high temperatures in an anaerobic condition with low humidity content. Feedstock’s generally required to ground into fine small particles to attain greater efficiency of thermal conversion in pyrolysis. Bio-oil is obtained as a result of pyrolysis process. The process of molecular thermal decomposition which occurs in the absence of oxygen or where the oxygen content is very low at atmospheric pressure is known as pyrolysis process. Bio oil is considered as an important source of renewable energy and a future substitute for fossil oil with potential for raw materials, manufacturing technology and business.
In present study, the biomass of peach seeds and seed kernels were characterized for use in pyrolysis process. The process of pyrolysis was conducted under different reaction conditions of temperature, particle size, reaction time and N2 flow rate to obtain the highest bio-oil yield. The bio-oil obtained after pyrolysis were also characterized for determination of physical and chemical properties [5].
Non-edible biomass
Non-edible biomass which has been selected for this research work was peach (also known as Prunus persica) seeds. “Swat local variety” of peach seeds was bought from Peshawar, KPK, Pakistan.
Seeds were dehulled and seed kernels were separated from dried seeds. Both the kernels and seeds were crushed by using a domestic grinder and sieved to obtain different sized particles ranging from 0.5 mm-2.5 mm with 0.5 mm interval. Both the feedstocks were then dried in an oven at 75°C for 3 days. The feedstock’s were then stored in proper sealed plastic bags for further use in pyrolysis.
Biomass characterization
The peach seed and seed kernel feedstock’s were characterized by proximate and ultimate analysis.
Proximate analysis: Both the peach seeds and seed kernels were characterized for their moisture, ash, volatile matter and fixed carbon contents according to ASTM D3172-07a standards.
Ultimate analysis: Ultimate analysis provides information for carbon, hydrogen, nitrogen, sulphur and oxygen contents of peach seeds and seed kernels analyzed from CHNSO elemental analyzer.
Pyrolysis experiments
The setup of pyrolyzer consists of a nitrogen gas supply, a reactor, vertical electrical furnace to heat the reactor, a condenser and a collector. Approx. 50 g of both the feedstocks, peach seeds and seed kernels, were fed into the reactor and closed it very tightly. Then heated the reactor by using an electrical furnace. The desired temperature was set in computer software in a control system. The temperature of the reactor was measured by K type thermocouple. All the experiments were performed and effect of different parameters such as temperature, particle size, reaction time, and N2 flow rate were investigated in this research. The particle sizes of peach seeds and seed kernelswere varied in a range of 0.5 mm, 1 mm, 1.5 mm, 2 mm and 2.5 mm, with the temperature maintained at 400°C, 450°C, 500°C, 550°C and 600°C and reaction times of 30, 60, 90, 120 and 150 (min) with N2 flow rates of 50 cm3/min, 100 cm3/min, 200 cm3/ min, 300 cm3/min and 400 cm3/min [6].
Once the process of pyrolysis started, the vapors coming out of the reactor’s outlet pipe were condensed by cooling water in a condenser and then collected in a collector. The liquid product obtained consists of water and pyrolytic oil. At the end, the liquid was transferred in a separating funnel to separate the oil from water by density difference. The resulting product obtained was bio oil.
The yield percentage of bio oil obtained is calculated by using the formula:
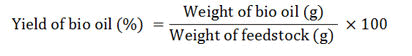
Bio oil characterization
Physical characterization: The peach seed and seed kernel bio oils obtained after pyrolysis process were characterized for physical properties by using ASTM standards. The moisture content in peach seed and seed kernel pyrolytic oil was determined by ASTM D6304 standard. The pH was estimated by using pH 210 microprocessor pH meter. The density was determined by ASTM D4052 standard. Viscosity was estimated by Ostwald viscometer using ASTM D445-11 standard. To determine the calorific value of peach seed and seed kernel bio oil, bomb calorimeter was used according to ASTM D4809-95 method [7].
Chemical characterization: To determine the presence of functional group of chemicals present in peach seed and seed kernel bio-oil, Fourier Transform Infrared Spectroscopy (FTIR) was used. On KBr disc, very small amount of bio oil was placed, and IR spectrum was observed in computer.
Biomass characterization
Biomass characterization is necessary to check its appropriateness for thermal process of pyrolysis. Peach seeds and seed kernels were characterized for proximate and ultimate analysis.
Proximate analysis: Table 1 shows the proximate analysis of peach seeds and seed kernels. This shows that both the peach seeds and seed kernels had less percentage of ash content and high percentage of volatile matter content. So, it could be good energy source. The moisture content of peach seed and seed kernel feedstocks was 8.5% and 7.5%. The ash content of peach seed and seed kernel feedstocks was 2.85% and 1.13%. The volatile matter content of peach seed and seed kernel feedstocks was 81.50% and 82.53%. The fixed carbon content of peach seed and seed kernel feedstocks which was determined by difference was 7.15% and 8.84% [8].
| Proximate analysis (Weight %) | Peach seeds | Peach seed kernels | Methods |
|---|---|---|---|
| Moisture content | 8.5 | 7.5 | ASTM D3173-11 |
| Ash content | 2.85 | 1.13 | ASTM D3174-00 |
| Volatile matter content | 81.5 | 82.53 | ASTM D3175-02 |
| Fixed carbon content | 7.15 | 8.84 | By difference |
Ultimate analysis: The ultimate analysis provides information about elemental composition of peach seeds and seed kernels shown in Table 2. It was conducted by using CHNSO elemental analyzer. The results showed that both the peach seeds and seed kernels had high percentage of carbon (61.01 and 47.13%) followed by oxygen (29.58 and 43.07) with hydrogen (4.29 and 7.16) with few amounts of nitrogen (3.98 and 1.88) and sulphur (1.14 and 0.76). This shows considerable difference in carbon and oxygen percentage with slight difference in hydrogen, nitrogen and sulphur percentage [9].
| Ultimate analysis (Weight %) | Peach seeds | Peach seed kernels |
|---|---|---|
| Carbon | 61.01 | 47.13 |
| Hydrogen | 4.29 | 7.16 |
| Nitrogen | 3.98 | 1.88 |
| Sulphur | 1.14 | 0.76 |
| Oxygen | 29.58 | 43.07 |
Pyrolysis experiment
The pyrolysis of peach seeds and peach seed kernels was performed in order to examine the optimal conditions at which the bio oil yield was maximum. Effect of four different parameters (temperature, particle size, reaction time and N2 flow rate) on production of bio oil was studied in present research work. Since the research was planned for production of bio oil, therefore bio char and gas were not examined further. The bio oils of peach seeds and peach seed kernels obtained after pyrolysis are represented in Figure 2 [10].
Temperature effect on yield of bio-oil: The experiments of pyrolysis were conducted to examine the influence of temperature variations on bio oil yields and optimum temperature at which yields were maximum. Figure 4 illustrates the temperature effect on yield of peach seeds bio oil. This shows that the yield of product increases gradually with temperature increase and maximum yield of 51% was obtained at 550°C temperature and then decreases with further increase in temperature. Bio oil yield decreases from 51% to 47% at 600°C.
The decrease in bio oil yield above 550°C can be attributed to more formation of non-condensable gases because of forceful cracking. High temperature in pyrolysis is linked with thermal cracking of vapors to generate more biogas and low bio oil yield (Figures 3 and 4).
Figure 4 illustrate the temp effect on yield of peach seed kernels bio oil. This shows that at lowest temperature (400°C) the process of disintegration was quite slow and bio oil yield obtained was 30%. Then increase in temperature increases the yield and maximum yield of 34.8% was obtained at 450oC temperature. Further increase in temperature decreases the yield and lowest yield (27%) was obtained at 600°C temperature.
Particle size effect on yield of bio-oil: Particle size of feedstock has significant impact on the yield of bio oil during pyrolysis process as it plays central role in mass and heat transfer. Changes in the size of feedstock particles greatly alter the heating rate which results in changing feedstock pyrolysis behavior. In present research, different particle sizes (0.5 mm, 1 mm, 1.5 mm, 2 mm, 2.5 mm) of peach seeds at 550°C temperature and seed kernels at 450°C temperature were pyrolyzed for 60 minutes. Figures 5 and Figure 6 shows the effect of particle size on bio oil yield of peach seeds and seed kernels. This shows that the yield initially increases with increase in size from 0.5 mm to 1 mm and then decreases with further increase in size [11].
Figure 5 shows that the highest peach seeds bio oil yield was 46.4% achieved by using 1 mm particle size while minimum yield (35%) was achieved with larger particle size of 2.5 mm. Figure 6 shows that the maximum yield of peach seed kernel bio oil was 41.5% obtained at 1 mm size of particles. The product yield decreases slowly from 41.5% to 30% at 2.5 mm particle size.
Reaction Time effect on yield of bio oil: The reaction time effect on bio oil yield of peach seeds and seed kernels was investigated in present research by performing pyrolysis process at 550°C temperature for seeds and at 450°C temperature for seed kernels using 1 mm particle size. Figures 7 and 8 illustrates the effect of different reaction times 30, 60, 90, 120, 150 (min) on bio oil yield of peach seeds and seed kernels. This shows that the bio oil yield increases with increasing reaction time of pyrolysis process from 30 min to 90 min.
In peach seeds, the bio oil yield increases from 32% to 43% by increasing the reaction time from 30 to 90 minutes by 30 minutes interval and then decreases with further increase in time. The bio oil yield reduced from 43% to 30% by increasing the reaction time from 90 to 150 minutes. In peach seed kernels the bio oil yield increases from 28% to 34.43% by increasing the reaction time from 30 to 90 minutes and then reduced to 25.7% at maximum reaction time of 150 min [12].
It was observed that the reaction time higher or lower than 90 minutes results in decreased yield of bio oil in both peach seeds and seed kernels. This may be due to inadequate time for pyrolysis of feedstock and high gas discharge rate. Thus 90 min was set as optimum time for pyrolysis process (Figures 7 and 8).
Nitrogen flow rate effect on yield of bio oil: The effect of nitrogen gas flow rate on bio oil yield of peach seeds and seed kernels was investigated in this research work by varying the flow rate (50, 100, 200, 300, 400 (cm3/min)) at 550 °C temperature for seeds and 450°C temperature for seed kernels using 1 mm particle size. Figures 9 and 10 demonstrates the effect of N2 gas flow rate on bio oil yield of peach seeds and seed kernels. These figures showed that the highest bio oil yield of peach seed was 43% obtained at 200 cm3/min N2 flow rate. While the highest yield of peach seed kernels was 33% obtained at 200 cm3/min N2 flow rate. They revealed the same trend between seeds and seed kernels as the bio oil yield initially increases up to 200 cm3/min and then decreases with further increase in N2 flow rate (Figures 9 and 10).
Bio oil characterization
Physical characterization: The peach seed and seed kernel bio oils obtained after pyrolysis process were characterized for physical properties by using ASTM standards. Table 3 shows the physical properties of peach seed and seed kernel bio oils [13].
The bio oils of peach seed and seed kernel contained 30% and 32% water which were determined by Karl Fischer titration. The pH of peach seed and seed kernel bio oils at room temperature was found to be 4.0 and 3.3. The value of density for peach seed and seed kernel bio oils at 15°C was 945.7 kg/m3 and 927.3 kg/m3. Both the peach seed and seed kernel bio oils are lighter than water as obvious from the density values.
The kinematic viscosity of peach seed and seed kernel bio oil was measured as 32.78 and 29.49 centistoke at 40°C. The gross calorific value of peach seed and seed kernel bio oils was found to be 35.09 MJ/kg and 27.62 MJ/kg. These calorific values show that the energy content of bio oil is very close to previous research findings (Table 3).
| Properties | Peach seeds | Peach seed kernel | Methods |
|---|---|---|---|
| Moisture content (%) | 30 | 32 | ASTM D6304 |
| pH | 4 | 3.3 | - |
| Density at 15°C (Kg/m3) | 945.7 | 927.3 | ASTM D4052 |
| Kinematic viscosity (Centistoke) at 40°C | 32.78 | 29.49 | ASTM D445-11 |
| Calorific value (MJ/Kg | 35.09 | 27.62 | ASTM D4809-95 |
Chemical characterization
FTIR: The FTIR spectrum of peach seed bio oil obtained at 550oC temperature, particle size of 1 mm, reaction time of 90 min. With 200 cm3/min N2 flow rate is shown in Figure 12. This shows 12 peaks in the spectrum at 2924.18, 2852.81, 2677.29, 1745.64, 1464.02, 1377.22, 1238.34, 1163.11, 1118.75, 1095.60, 1031.95 and 723.33 (cm-1) wavelengths. The FTIR spectrum of peach seed kernel bio oil obtained at 450°C temperature, particle size of 1 mm, reaction time of 90 min. with N2 flowrate of 200 cm3/min is shown in Figure 11. This shows 14 peaks in the spectrum at 3477.77, 2924.18, 2852.81, 2677.29, 2316.58, 2044.61, 1745.64, 1454.38, 1377.22, 1240.27,1165.04, 1099.46, 914.29 and 723.33 (cm-1) wavelengths (Figures 11 and 12) [14].
Table 4 shows several peaks with different intensity of different types of bond present in peach seed and seed kernel bio oil. The bond stretching of O and H between 3600 cm-1 and 3200 cm-1 represents the existence of polymeric OH and other water impurities. The bond stretching of C and H in the range of 2960 cm-1 to 2850 cm-1 represents the presence of alkane. The C=O stretching in 1760 cm-1-1670 cm-1 wavelength range shows the occurrence of aldehyde, ketone, carboxylic acid, ester groups. The absorption peak of 1470 cm-1-1350 cm-1 shows C=C bond stretching indicating the presence of alkyne functional group. The peak observed from 1420 cm-1-1330 cm-1 is due to bending of O and H and shows the alcohol existence. The C-O bond stretching between 1320 cm-1 to 1000 cm-1 represents the existence of ether and ester. Likewise, the absorbance peak between 900 cm-1-650 cm-1 with bending of O and H shows the presence of aromatic group. The composition of functional group analysis shows that peach seed and seed kernel bio oils mainly contain carboxylic acid, ester, ether, alkane, alcohol and aromatic groups (Table 4) [15].
| Wavelength range (cm-1) | Frequency (cm-1) | Type of vibration | Functional group |
|---|---|---|---|
| 3600-3200 | 3477.77 | O-H stretching | Polymeric OH, water impurities |
| 2960-2850 | 2852.81, 2924.18 | C-H stretching | Alkanes |
| 1760-1670 | 1745. 64 | C=O stretching | Aldehyde, carboxylic acid, ketone, ester |
| 1470-1350 | 1454.38 | C-C stretching | Alkyne |
| 1420-1330 | 1377.22 | O-H bending | Alcohol |
| 1275-1200 | 1240.27 | C-O stretching | Ether |
| 1320-1000 | 1099.46, 1165.04 | C-O stretching | Carboxylic acid, alcohol, ester, ether |
| 950-910 | 914.29 | O-H bending | Carboxylic acid |
| 900-650 | 723.33 | O-H bending | Aromatic rings |
In present research the potential of using peach seeds and seed kernels as non-edible biomass for the production of bio oil through pyrolysis process have been investigated. The process of pyrolysis was conducted and effect of temperature, particle size, reaction time and N2 flow rate have been examined. The highest bio oil production of peach seed was 51% while peach seed kernel was 41.5% obtained at 550°C and 450°C temperature, 1 mm of particle size, 90 minutes of reaction time, and 200 cm3/min of N2 rate of flow. This showed that the bio oil yield of peach seed is comparatively higher than seed kernel bio oil, therefore peach seed is better feedstock for the production of bio oil.
Now a days, needs for fossil fuels as an energy source are rising during the period of industrial revolution. With this situation, scientists trying to find alternatives to make sure the adequate supply of energy. Bio oil is the effective choice for replacing the energy source in the form of renewable energy.
[Crossref] [Google Scholar] [PubMed]
Journal of Environmental Analytical Chemistry received 1781 citations as per Google Scholar report