Case Report - (2022) Volume 8, Issue 4
Received: 01-May-2022, Manuscript No. ELJ-22-62373;
Editor assigned: 04-May-2022, Pre QC No. ELJ-22-62373(PQ);
Reviewed: 19-May-2022, QC No. ELJ-22-62373;
Revised: 01-Jul-2022, Manuscript No. ELJ-22-62373(R);
Published:
18-Jul-2022
, DOI: 10.37421/ 2472-0895.2022.8.166
Citation: Rafati, Mohammad Reza and Ehsan Yousefi
Mazhin. "Interpretation of Serum Valproate Level in Complicated
Patient: A Case Report." Epilepsy J 8 (2022): 166.
Copyright: © 2022 Mazhin EY, et al. This is an open-access article distributed under the terms of the creative commons attribution license which
permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Valproate is an antiepileptic drug which is commonly used for the treatment of focal and/or generalized epilepsy and mood disorders. We have reported a case of 10-years-old, 30 kg boy was admitted in the pediatric intensive care unit due to status epilepticus and then was received some antiepileptic drugs including valproate. Almost two weeks after starting valproate, the total serum level of valproate was measured that was very low. We investigated the cause of low valproate levels in this patient.
Valproate • Hypoalbuminemia • Meropenem • Status epilepticus • Drug-drug Interaction
AEDs: Antiepileptic Drug; t: time; D: Dose; V is Volume of Distribution; K is the Elimination Rate Constant; τ; Dosage Interval; PICU: Pediatric Intensive Care Unit; BID: Twice a Day; TDS: Three Times a Day; C: Concentration; N: Normalized
Valproate is an antiepileptic drug which is commonly used for the treatment of focal and/or generalized epilepsy and mood disorders. Valproate is an Antiepileptic Drug (AED) that is chemically related to free fatty acids and is used in the treatment of generalized, partial, and absence (petit mal) seizures. As such, it has the widest spectrum of activity compared to the other currently available antiepileptic drugs.
A 10-year-old, 30 kg boy was admitted in the Pediatric Intensive Care Unit (PICU) due to status epilepticus and then was received midazolam infusion, phenobarbital IV 60 mg twice a day (BID) and valproate IV 600 mg BID to control seizure. After a few days, the patient was treated with meropenem IV 450 mg BID, ciprofloxacin IV 300 mg BID and vancomycin IV 500 mg TDS for ventilator associated pneumonia. One week after the initiation of meropenem, the total serum level of valproate was measured, which was equal to 4.4 mg/L.
Valproate is an Antiepileptic Drug (AED) that is chemically related to free fatty acids and is used in the treatment of generalized, partial, and absence (petit mal) seizures. As such, it has the widest spectrum of activity compared to the other currently available antiepileptic drugs [1,2]. Now available in intravenous, as well as oral form, valproate can be used for the acute treatment and chronic prophylaxis of seizures [3,4]. Valproate is also a useful drug for the treatment of bipolar affective disorders and the prevention of migraine headaches [5].
The generally accepted therapeutic range for total valproate steady-state concentrations is 50–100 mcg/mL, although some clinicians suggest drug concentrations as high as 175 mcg/mL with appropriate monitoring of serum concentrations and possible adverse effects.
For estimation of steady state total valproate concentration, we used the below equation:
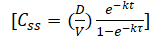
Where C is drug serum concentration at time t, D is dose, V is volume of distribution, K is the elimination rate constant and is the dosage interval.
The clearance rate for a child who takes other drugs that induce hepatic drug metabolism (phenobarbital in our case) is 20–30 mL/h/kg. Using a value of 25 mL/h/kg, the estimated clearance would equal 0.75 L/h: Cl=30 kg* 25 mL/h/kg=750 mL/h or 0.75 L/h. Using 0.2 L/kg, the estimated volume of distribution would be 6 L: 30 kg* 0.2 L/kg = 6 L.
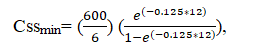
But in our case, the measured minimum concentration of valproate in steady state was 4.40 mg/L. There are some reasons for this significant difference in estimated and measured valproate concentration in our patient that is described in below.
In the case of drugs with high protein binding, it may be preferable to determine their free concentration; however, in clinical laboratories, the standard procedure is to monitor the total drug concentration, due to technical difficulties and a lack of established reference ranges for free drug concentrations [6].
Valproate is highly protein bound to albumin with typical values of 90-95% and the drug-protein binding depends on both the serum albumin concentration and the drug concentration. Free fraction of valproate increases in hypoalbuminemia. If the amount of total concentration of valproate is measured, we should normalize the total concentration of valproate according to the concentration of serum albumin. The total concentration of valproate is normalized using the below equation:
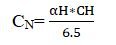
where is the free fraction of the drug corresponding to the patient’s particular albumin concentration and CH is the total concentration of valproate.
The serum albumin concentration in our patient was 32 g/L, and the free fraction of valproate in this albumin concentration is 13%, thus the normalized minimum total concentration of valproate in steady state in our patient is 8.80 mg/L: CN=4.40*13/6.5=8.8. There is still a significant difference between expected concentration and measured concentration of valproate after the normalization of valproate concentration. Therefore, another factor still exist that caused to decrease in measured total serum concentration of valproate (Table 1).
| Albumin (mg/L) |
|---|
| 4.2 |
| 4.1 |
| 4.0 |
| 3.9 |
| 3.8 |
| 3.7 |
| 3.6 |
| 3.5 |
| 3.4 |
| 3.3 |
| 3.2 |
| 3.1 |
| 3.0 |
| 2.9 |
| 2.8 |
| 2.7 |
| 2.6 |
| 2.5 |
| 2.4 |
| 2.3 |
| 2.2 |
| 2.1 |
| 2.0 |
| 1.9 |
| 1.8 |
Carbapenems have been shown to reduce serum concentration of valproate in case reports and retrospective studies. The interaction was first reported in the Japanese literature in the late 1990’s. The mechanism behind the interaction is complex and still has not been fully elucidated. The reduction in serum valproate concentration induced by carbapenems happens rapidly within 24 hours of concomitant administration and may lead to the aggravation of seizures. The interaction between carbapenems and valproate may result in a significant decrease in serum valproate concentrations. The concomitant use of meropenem and valproate caused a 77-78%reduction in valproate concentrations. A reduction in valproate concentrations was seen in patients using ertapenem or imipenem concomitantly. The median reduction of valproate concentrations was 68% when used in combination with ertapenem, and the combined use of valproate and imipenem reduced valproate concentrations by 50%. In our patient, the total normalized concentration and expected concentration of valproate are 8.8 mg/L and 28.7 mg/L, respectively.
Administered meropenem in our patient caused decrease in valproate concentration by 70%.
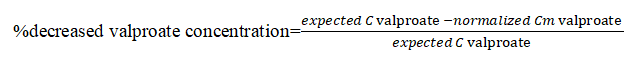
Plasma protein binding of valproate is saturable within the therapeutic range, which results in less protein binding and higher unbound fraction of drug at higher concentrations. The concentrationdependent protein binding of valproate causes the drug to follow nonlinear pharmacokinetics. In the case of valproate, when the dose is increased total drug steady-state concentration increases less than expected, but unbound steady-state drug concentration increases in a proportional fashion (e.g., when the dose is doubled, total serum concentration increases 1.6–1.9 times but unbound steady-state serum concentration doubles).
More information is available that identifies the clinical situations where unbound valproate serum concentration measurement is useful. As is the case with phenytoin, measurement of unbound valproate serum concentrations should be considered in patients with factors known to alter valproate plasma protein binding. These factors fall into three broad categories:
1. Lack of binding protein where there are insufficient plasma concentrations of albumin,
2. Displacement of valproate from albumin binding sites by endogenous compounds, and
3. Displacement of valproate from albumin binding sites by exogenous compounds.
In our case, three factors were suspected to decrease the total concentration of valproate including hypoalbuminemia and concomitant administration of phenobarbital and meropenem. Phenobarbital has been shown to be capable of significantly increasing the clearance of valproate. The mechanism of this possible interaction is likely phenobarbital-mediated induction of valproate metabolism (via glucuronidation and CYP-mediated oxidation). With regard to pharmacokinetics, valproate typically has high albumin binding (90-95%), but it may be reduced in specific situations, including the occurrence of hypoalbuminemia and azotemia, or the administration of medications that compete for binding, such as aspirin and phenytoin. valproate follows nonlinear pharmacokinetics owing to saturable, or concentration-dependent, plasma protein binding. This is the type of nonlinear pharmacokinetics that occurs when the number of drug molecules overwhelms or saturates albumin’s ability to bind the drug in the plasma. When this occurs, total steady-state drug serum concentrations increase in a disproportionate manner after a dosage increase, but unbound steady-state drug serum concentrations increase in a proportional fashion. Although monitoring of free valproate serum concentration is recommended, it is not available in many institutions. Only approximately 2% of laboratories routinely provide free valproate monitoring in the United States. An albuminbased normalizing formula was developed as an alternative method of monitoring valproate accurately.
Serum valproate concentrations is decreased significantly after the introduction of carbapenems. Among the carbapenems used, a stronger interaction was observed between meropenem and valproate than that between ertapenem and imipenem. The reduction of valproate concentrations was seen within 24 hours of the initiation of treatment with either high or low doses of carbapenems. Most reports suggest that combination therapy with valproate and carbapenems should be avoided, unless necessary. When simultaneous use is inevitable, other AEDs should be temporarily added during the co administration of valproate and carbapenems. The addition of another AED should be maintained for at least 2 weeks after the discontinuation of carbapenems because it takes 1-2 weeks for serum valproate concentrations to return to therapeutic concentrations. The effect of increasing valproate dose, via “oral or intravenous” routes or “loading or maintenance,” did not help in achieving therapeutic concentrations. Levetiracetam could be a good choice because of the low risk of drug interactions and the rapid titration protocol.
Not applicable.
Not applicable.
The authors declares that he has no competing interests.
Epilepsy Journal received 41 citations as per Google Scholar report