Research Article - (2024) Volume 11, Issue 1
Received: 17-Mar-2023, Manuscript No. IJN-23-92111;
Editor assigned: 22-Mar-2023, Pre QC No. IJN-23-92111 (PQ);
Reviewed: 05-Apr-2023, QC No. IJN-23-92111;
Revised: 29-May-2023, Manuscript No. IJN-23-92111 (R);
Published:
05-Jun-2023
, DOI: 10.37421/2376-0281.2023.10.527
Citation: Sharma, Anjali. "Effectiveness of Coma Stimulation Program in Traumatic Brain Injury." Int J Neurorehabilitation Eng 10 (2023): 527.
Copyright: © 2023 Sharma A. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Background and purpose: Coma is the prolonged period of unconsciousness immediately following traumatic brain injury. Coma is one of the results of brain injuries on the other hand; sensory deprivation is one of the complications, which have a high risk in Intensive Care Unit (ICU) wards for these patients. Cause of nature of illness they are keeping in emphatic environments that it could lead to decreasing sensory inputs and in this condition brain does not have a normal level of brain activity and consequently would lead to sensory deprivation. The study was done to find out the effectiveness of coma stimulation program in traumatic brain injuries.
Design and setting: A randomized control trial study. The study was conducted at Sri Aurbindo Institute of Medical Sciences Indore.
Subjects: The traumatic brain injury patients who admitted at study center and further met the inclusion criteria selected as subjects during specified schedule. A total of 15 traumatic head injury patients who were diagnosed as specified types of traumatic brain injury were purposively selected from the in-door patient department at Sri Aurobindo institute of medical sciences. To all the subjects one session of coma stimulation program with two sessions of conventional physiotherapy were given for 4 weeks.
Outcome measure: Glasgow coma scale.
Results: Statistical analysis results showed significant improvement in level of consciousness in all the cases of traumatic brain injury received coma stimulation program. Frontal contusion injuries showed higher gain followed by temporoparietal contusion and parietal contusion injuries.
Conclusion: The results suggest that coma stimulation program is better treatment in frontal contusion injuries followed by temporoparietal and parietal contusion injuries.
TBI • Arouasl • Coma • GCS • Brain injuries
The brain injury association of America describes a TBI as “an alteration in brain function or other evidence of brain pathology, caused by an external force”. The brain and supporting structures are extremely vulnerable to traumatic injury [1]. A report from the British society of rehabilitation medicine defined TBI as “brain injury caused by trauma to the head, including the effects of direct complications of trauma, notably hypoxemia, hypotension, intracranial hemorrhage and raised intracranial pressure”. In the codes of the International Classification of Diseases (ICD-10) specifying clinical features of brain injury, skull fractures, brain concussions, brain contusions, and other intracranial injuries, including subarachnoid, subdural, and extradural hematomas and diffuse injuries, are listed. In the present work, TBI is defined as damage of brain tissue caused by external mechanical force. Patient with TBI are often left with significant physical, cognitive, and behavior squalae requiring prolonged hospitalization and the need for post-acute rehabilitation program [2]. The center of disease control and prevention is referred to TBI as the silent epidemic. High age is one of the risk factors, which is effective on the severity of disease after TBI (Figure 1).
Incidence: Population based studies in the US suggest that the incidence of TBI is between 180-250 per 100,000 populations per year incidence may be higher in Europe and South Africa. There are groups at high risk for TBI. It includes males and individuals living in regions characterized by socioeconomic deprivation. There are selective age groups at risk for TBI. This includes the very young, adolescents and young adults and the elderly [3]. Epidemiological studies have shown age-specific incidence of hospital-treated TBI, with highest rates in persons younger than four and older than 65 years. According to national and international studies, TBI occur in males about twice as often as in women. Falls are the leading cause of injury in Scandinavia and in the US, followed by traffic accidents, assaults and other injuries. However, traffic accidents are the leading cause of injury in Southern Europe, and continue to be the main cause of severe and fatal injuries.
Assaults are common cause of injury in males and associated with substance influence at the site of injury. In Europe, about 70%-80% of patients are classified with mild TBI based on the Glasgow Coma Scale (GCS), and moderate and severe TBI with 10% each. In a recent Norwegian study the ratio of hospitalized patients with severe, moderate and mild TBI was 1:1.3:14. Approximately 10%-15% of patients have more severe injuries requiring specialist care. Severe TBI is associated with high mortality rates among hospitalized patients (30%-50%). How ever, the overall rate of TBI mortality has generally decreased since the 1980’s, and can be attributed to improved emergency and acute trauma services [3].
TBI classification
The most commonly used clinical indices of TBI severity for adults is the Glasgow Coma Scale (GCS) assessing level of consciousness after TBI based on eye opening, motor and verbal responses. Mild TBI is defined as a state with GCS scores of 13-15, moderate 9-12 and severe 3-8. Variation in the definition of mild TBI exists, and Scandinavian guidelines and recent studies defined moderate TBI as a GCS score of 9-13. Other researchers suggest that persons with GCS score of 13-15 and intracranial lesions should be classified as moderate TBI because they have a similar course of recovery to those with GCS score of 9-12. Other scales that assess extra cranial injuries and physiological instability are the Abbreviated Injury Scale (AIS) (Association for advancement of automotive medicine) and Injury Severity Score (ISS). The AIS defines the severity of injury in different body regions, while the ISS quantifies the severity of multiple body region injuries based on the AIS codes. A new version of the ISS, the New ISS (NISS), exists and takes the three most severe AIS values irrespective of body region in full account of multiple injuries in the same body region. In many TBI studies, description of the location or anatomical features of TBI in acute phase is done by the Marshall score for Computed Tomography (CT) findings [4]. It classifies the presence or absence of a mass lesion and differentiates diffuse injuries by signs of increased intracranial pressure. A more recent and standardized CT based classification is the Rotterdam score combining intracranial CT findings to predict outcomes. Magnetic Resonance Imaging (MRI) provide information about the neuroanatomical of the skull, brain tissue, and blood vessels and assess the extent of brain injury and the medical sequelae of traumatic brain injury (edema, intracranial bleeding, degeneration). MRI has often been used for diagnostic purposes in follow-up studies for detecting Diffuse Axonal Injuries (DAI). DAI is damage to the axons due to shearing, acceleration declaration, and rotational forces on the brain. It is classified into 1-3 stages of DAI;
• Lesions confined to the lobar white matter.
• Callosal lesions.
• Lesions in the dorsolateral brainstem.
In addition, the presence and duration of Posttraumatic Amnesia (PTA) are often used as tools for classifying TBI. The term PTA is defined as the “time elapsed from injury until recovery of full consciousness and the return of ongoing memory” or as a period of clouded consciousness which precedes the attainment of full orientation and continuous awareness in persons recovering from head injuries. Persons in PTA suffer from anterograde amnesia, i.e. an inability to remember new experiences, and they often have a period of retrograde amnesia, i.e. loss of memory of events before injury. TBI severity also classified as defined by the American Congress of Rehabilitation Medicine (ACRM). Mild TBI was defined by an initial GCS score of 13-15, change in mental status without Loss of Consciousness (LOC), or LOC up to 30 minutes and PTA up to 24 hours. Moderate TBI was defined by GCS score of 9-12, LOC more than 30 minutes, but less than 6 hours. Severe TBI was defined by GCS score of #8 and LOC more than 6 hours. In the ACRM and WHO definitions, no minimal PTA duration is specified, and PTA duration of a few seconds qualifies. But if PTA duration exceeds 24 hours TBI should no longer be considered as mild. This ACRM definition has been widely used, especially in the field of rehabilitation and neuropsychology [5].
TBI rehabilitation
Rehabilitation following TBI developed during World Wars I-II with the identification of neurocognitive and affective disorders by Poppelreuter, Goldstein, Russel and Luria. In the 1950’s and 1960’s, TBI rehabilitation concerned motor disorders within mechanical and orthopedic frameworks, while behavior-conditioning and psychoanalysis started under the influence of behaviorism. In the 1980’s, holistic programs and cognitive neuropsychology developed. Rehabilitation after TBI has derived from these works and focus on more home-based therapy in ADL and work skills. TBI induces disturbances in different domains (physical, cognitive and social abilities) at different levels. Further, it involves disruption in the course of psychic state and the life plan of the person and often changes in personality and behavior [6]. The main goal of rehabilitation is to improve functional independence, re-entry to a community and return to work, as well as quality of life. Rehabilitation has been described as a reiterative, active, educational, problem solving process focused on a patient’s disability with assessment, goal setting, intervention and evaluation. Rehabilitation following TBI is a continuous process that involves the identification of problems and needs, implementation of adequate interventions and evaluation of outcome. TBI rehabilitation is generally long consisting of three phases: Acute rehabilitation in the trauma hospital followed by sub-acute (generally inpatient) rehabilitation, and post-acute outpatient rehabilitation. Acute rehabilitation aims to reduce complications that can occur as a result of injury and promote functional recovery through multi-sensory stimulation. Sub-acute inpatient rehabilitation facilitates and accelerates recovery of physical and cognitive impairments, and compensates for disabilities. Post-acute rehabilitation includes outpatient therapy for physical, domestic and social independence, reduction of handicaps and re-entry to the community. Rehabilitation after TBI are organized in relation to these phases and are often carried out by a specialized brain injury rehabilitation team [7]. The team is multidisciplinary and works on common goals for each patient, involves and educates the patient and family, has relevant knowledge and skills, and resolves most of the common problems faced by the patient.
Mechanism of brain injury
In 1966, Goldsmith classified three main mechanical causes of head injury:
Pathophysiology of traumatic brain injury
Acceleration, deceleration, and rotational forces as well as penetrating objects act to cause tissue laceration, compression, tension, shearing or a combination resulting in primary injury. Imaging permits the categorization of brain damage into focal and diffuse, although often both types coexist. Alternatively brain damage can be classified as primary occurring or impact, or secondary from ongoing neuronal damage, hematoma, brain swelling, ischemia or infection [8].
Focal damage
Focal brain injury is localized to the area of the brain under the site of impact on the skull. Damage in the form of a hematoma, edema, contusion, or laceration or a combination of four. Cortical contusion and laceration:
These may occur under or opposite (counter-coup) the site of impact, but most commonly involve the frontal and temporal lobes. Contusion and laceration can also injure the cranial nerves, laceration of Dura and/or arachnoid may cause cerebrospinal fluid to discharge from nose [ 9].
Intracranial hematoma
Intracranial bleeding may occur either outside (extradural) or within the dura (intradural). Intradural lesions usually consist of a mixture of both subdural and intracerebral hematomas although pure subdural occur in a proportion.
Intracerebral and subdural
Contusion in frontal and temporal lobes often lad to bleeding into the brain substance, usually associated with an overlying subduarl haematoma.
Tentorial nad tonsilar herniation
It is unlikely that high intracranial pressure alone directly damage neuronal tissue, but brain damage occur as a result of tonsillar or tentorial herniation [10].
Infection
Infection secondary to open wounds. Infection in brain tissue may cause swelling and cell-death.
Diffuse damage
Diffuse axonal injury: Shearing force cause immediately mechanical damage to axon. Axonal changes eventually lead to their separation from the soma. Diffuse axonal injury may be severe enough to result in coma. In milder form, more spotty lesions are seen, including deficits such as memory loss, concentration difficulties, decreased attention, headaches, sleep disturbances, and seizures.
Impairment associated with traumatic brain injury
Neuromuscular impairments consist of abnormal tone, sensory impairments, impaired balance can occur, cognitive impairment like altered level of consciousness, memory loss, visual impairment and perceptual impairments present [11]. Behavioral problem associated with it. Communication impairments like receptive aphasia, expressive aphasia, dysarthria, auditory deficits, may be present.
Coma
Coma is the prolonged period of unconsciousness immediately following traumatic brain injury. Prolonged coma and vegetative state follow severe closed head injury assessed at discharge from a trauma centre. According to the traumatic coma data bank 52% of vegetative survivors from severe head trauma regain consciousness within one year post-injury and 40% improve to a higher glasgow outcome scale within six months (task force on PVS). The remaining individuals die or remain in a vegetative state for months or years. According to Jenett and Teasdale, coma is defined as not obeying commands, not uttering words, and not opening eyes. The glasgow coma scale score can be used to identify coma, with a score of 8 or less defining coma [12].
Measurement of coma
Depth and duration of coma have long been viewed as the most useful indicant of brain damage. To characterize the continuum of coma by practical and reliable procedure. Teasdale and Jenett developed the Glasgow coma scale evaluate three component of wakefulness independently of each other.
Coma stimulation program
Coma stimulation program is the foundation of rehabilitation because a coma is viewed a state of decreased responsiveness to the environment related to sensory deprivation. These programs are based on the principle of brain plasticity and of sensory deprivation. The idea according to which an injured brain has the capacity to reorganize itself to compensate for the affected region is broadly accepted for several years. The practical implication of sensory deprivation is that controlled stimulation (consisting of auditory, gustatory, olfactory, tactile, kinesthetic, and visual) may meet the higher threshold of the reticular neurons and increased cortical activity or that the undamaged axons may actually send out collateral connection called collateral spouting which assist in reorganizing the brain's activity [13].
Traumatic brain injury
Jamshid Ghajar conducted a review on traumatic brain injury concluded that advances in critical care imaging and the reorganization of trauma system have led to a pronounced reduction in death and disability resulting from traumatic brain injury. This improvement has resulted largely from early recognition and treatment of cerebral hypoperfusion. G Gururaj presented a paper on Epidemiology of traumatic brain injuries; Indian scenario: Concluded that Traumatic Brain Injuries (TBIs) are a leading cause of morbidity, mortality, disability and socioeconomic losses in India and other developing countries. It is estimated that nearly 1.5 to 2 million persons are injured and 1 million succumb to death every year in India [14].
Masson, Trancoise MD conducted a prospective study on severe brain injuries; concluded that a decrease in severe TBI incidence when results are compared with another study conducted 10 years earlier in the same region. This is because of a decrease in traffic accidents. However, this results in an increase in the proportion of falls in elderly patients and an increase in the proportion of falls in elderly patients. This increased age influences the mortality rate. Steriade M; presented a paper on “Arousal: Revisiting the reticular activating system”. Examined the anatomical details of the reticular activating system and concluded its function as regulating arousal and sleep-wake transition.
Centers for disease control and prevention presented morbidity and mortality weekly reports suggests that there is a 22% decline in the TBI-related death rates from 24.6/100,000 U.S. residents in 1979 to 19.3/100,000 in 1992. Firearm related rates increased 13% from 1984 to 1997, underlining a 25% decline in motor vehicle related rates. These data highlight the success of efforts to prevent such injury due to firearms. Eastman, Peggy, in his study on what is known about gender, race and the demographics of TBI. They found out that 16% of vetrans are women and they tend to have a lower incidence of TBI, than men. Estrogen and progestrone may have neuro-protective properties because of hormone's excitatory pathways. They have also suggested that regardless of a particular gender, ethnicity or socioeconomic status, TBI is a lifelong situation, especially severe TBI.
A randomized control trial, on prognosis after SDH OR EDH in a series of 171 patients suffering acute SDH or EDH after closed TBI. The rate and the grade of clinical recovery were evaluated. They found out that overall mortality in acute SDH was 57% in acute EDH 25% and full recovery and minimal neurological deficits being 23% and 58% respectively. Iimari Asikainen, et al., conducted a study on predicting late outcomes for patients with TBI referred to a rehabilitation program: In study of 500 patients 5 year or more after injury concluded that the extent of recovery and quality of life for rehabilitation patients with TBI can be estimated early on by prognostic factors reflecting injury severity in the acute phase [15].
Glasgow coma scale
GCS staff completed scale for assessing depth and duration of impaired consciousness and coma recorded on a staff complete chat. There aspects of behavior are recorded, motor responses and eye opening, each charted on a 5 point scale in a graphical form, research shows high level of consistency between different observers. Mchelle R Gill, et al., conducted a prospective observational study on Interrater reliability of GCS in the emergency department. They found that a moderate degree of interrater agreement for the GCS and its component.
Menegazzi, et al., conducted a prospective sequential trial to find out reliability of the glasgow coma scale when used by emergency physicians and paramedics; determine the reliability of the GCS concluded that the GCS shows statistically significant reliability between emergency physician and emergency medical technician paramedics. It also has a significant level of interpreter reliability. Juarez, Valerie J, et al., conducted a study on Interrater reliability of the glasgow coma scale. The purpose of this study was to test the interrater reliability of the Glasgow Coma Scale (GCS) when used in assessing neurologically impaired patients found a moderate to high agreement rating demonstrating that this tool has interrater reliability [16].
Fielding K , Rowley G conducted as study for reliability of assessments by skilled observers using the glasgow coma scale: In this study a very high degree of inter rater reliability was demonstrated between Registered Nurses (RNs) who were educated and experienced in use of the scale. Kameshwar Prasad conducted a study on the glasgow coma scale: A critical appraisal of its clinimetric properties; concluded that the scale has a good sensibility and reliability. It has a well-established cross sectional construct validity. Its predicative validity in traumatic coma, when combined with age and brain stem reflexes in good in the generating sample [17].
Amando Alejandro Baez, et al., conducted a cross sectional study on Precision and reliability of GCS score among a cohort of Latin America pre hospital emergency care provider proved the GCS is the standard measure to quantify the level of consciousness in TBI cases. Edward SL; Br Journal of nursing conducted a study on using the glasgow coma scale: Analysis and limitations. This article addresses the gap between the literature and practice in relation to the use of the GCS. It will explore level of consciousness and GCS. The instigation of both central and peripheral painful stimuli is analyzed in an effort to prevent ritualistic practice. Attention is also given to the importance of including vital signs when using the GCS, as these can tell a lot, if not more, about the patient’s neurological condition. Finally the limitation of the GCS are examined to assist in a more accurate assessment tool for neurologically impaired patient.
TBI induced coma
Douglas I Katz, et al., conducted a cohort study on; predicting course of recovery and outcome for patient admitted to rehabilitation: Concluded that post traumatic amnesia had a clear predictable relationship to length of coma in diffuse axonal injury [18].
Albino Bricolo, MD, et al., in his review on prolonged post traumatic unconsciousness therapeutic assets and liabilities concluded that prolonged coma following severe head injury is a serious condition because it implies a poor proved steps gnosis. Patients emerge from unconsciousness in consecutive steps representing the restoration of increasingly complex neurological function; the timing of these steps is very variable and sometimes covers the several months.
Coma stimulation program
Gillion A conducted an observational study on Snoezelen: A controlled multi-sensory stimulation therapy for the children recovering from severe brain injury; concluded that a beneficial use of snoezlen therapy with children recovering from severe brain injury. R Jones, et al., conducted a case study on auditory stimulation effect on a comatose survivor of traumatic brain injury concluded that providing familiar auditory stimulation programs for coma patients in the ICU could be effective. Poornnipa Urbenjaphol conducted a quasi-experimental study on effect of sensory stimulation program on recovery in unconscious patients with traumatic brain injury. Concluded that sensory stimulation program can enhance brain recovery in traumatic brain injured patient [19]. Traumatic head injury: Early intervention by coma arousal therapy has significant effect on CRS in Traumatic head injury patients when compared to the patients who did not receive coma arousal therapy.
M. Lippert Gruner, D. Terhaag conducted a study on Multimodal Early Onset Stimulation (MEOS) in rehabilitation after brain injury: Concluded that an early and consistent administration of the correct rehabilitation program is of crucial importance for the restoration and improvement of cerebral function, as well as social reintegration. The most significant changes were caused by tactile and acoustic stimulation. Coma arousal therapy concluded that from families of 38 patients in Sydney, Australia who were involved in CART show that 84% reported that overall impact of the program on them was good. 79% of families felt that the effect on would advise other people to be involved in such a program. Sylvia Mitchell, et al., conducted an experiment study on a therapeutic intervention in the treatment of head injury: Concluded that the total duration of coma was significantly shortened and that coma lightened more rapidly for the experimental group. Sonouski Cheryl conducted a study on early intervention: Coma stimulation in the intensive care unit concluded that an individual of recovery from brain injury is paramount in stimulating the reticular activating system and promoting brain reorganization.
Sarah L Wilson conducted a review of the evidence for the effectiveness of sensory stimulation treatment for coma and vegetative state concluded that sensory stimulation can alter behavior in the unconscious patient and can reduce the duration of acute coma. Jiang JV, et al., conducted experimental study on Effect of arousal methods for 175 cases of prolonged coma after severe traumatic brain injury and its related factors concluded that application of appropriate arousal procedure in person’s recovery of consciousness in patients with prolonged coma. Mary E Hall, et al., conducted a pilot study single subject design on the effectiveness of directed multisensory stimulation versus non-directed stimulation in comatose CHI patients: They have given alternating weeks of directed multisensory stimulation (SDS) for half an hour a day in an ABAB single subject design. Eye movement, motor and vocal response to stimuli were recorded. Rancho scale appeared to indicate a greater degree of responsiveness to the SDS compared to NDS treatment [20].
Aim: To study the effect of coma stimulation in traumatic brain injury of different sites.
Objective: To study the effect of coma stimulation in traumatic brain injury affecting different sites of brain any patients admitted in the neurosurgery department of SAIMS in the study duration of 12 months.
Dependent variable: GCS Score
Independent variable:
• Conventional physiotherapy
• Multisensory stimulation
Hypothesis
Null-hypothesis: Coma stimulation program may not beneficial for the patients of all varieties of traumatic brain injury.
Alternate hypothesis: Coma stimulation program may beneficial for the patients of all varieties of traumatic brain injury.
Study design: Pre and post experimental design.
Sampling method: Convenient sampling.
Inclusion criteria:
• Acute traumatic brain injury patient.
• Glasgow coma scale score: 5 to 8.
• Gender: Both male and female.
• Age group: 25 to 50.
• Post-surgical cases.
Exclusion criteria:
• Patient with congenital hearing loss.
• Patient with congenital visual loss.
• Patient on ventilator.
• Open wound and skin abrasions.
• Intubated patient.
Study set up:
• IPD Bhandari hospital and research centre.
• Neurosurgery Department (IPD), SAIMS.
• Neuro ICU, SAIMS.
Study duration
The total duration of study was 1 year. Individual subject management was done for a period of 4 weeks with treatment time of 30-45 minutes for conventional treatment in two sets along with another session of 50 min for multisensory stimulation therapy for 6 days a week.
Procedure: Patients were selected according to the inclusion criteria. After taking consent from the department they had been assessed on day 1 using patient assessment chart and by GCS. Total 15 patients were selected and had been divided into seven groups.
• Frontal contusion.
• Frontotemporal contusion.
• Temporoparietal contusion.
• Frontal temporoparietal contusion.
• Parietal contusion.
• Temporal contusion.
• Diffuse axonal injury.
The level of consciousness was assessed by GCS on day 1st and lastly on day 30th of the treatment. To the all seven groups two sets of conventional physiotherapy were given which were interspaced by 6-7 hours and one session of multisensory stimulation were given.
Conventional physiotherapy
• Proper positioning.
• Chest physiotherapy.
• ROM exercises-gentle PROM for all four limbs.
• Once the patient is medically stable-stretching to prevent
contracture and deformities due to muscle spasm hypertonicity
tightness.
• Gradually progress to mat exercises.
• Supine to side lying.
• Sidelying to sitting.
• Sitting to standing with support (tilt table).
• DVT prevention.
• Proper positioning in wheel chair.
• Balance training.
To the experimental group one session of conventional physiotherapy and one session of multisensory stimulation were given.
Multisensory stimulation
Sensory stimulation was provided to the patient through following different modalities.
• Auditory.
• Visual.
• Olfactory.
• Tactile.
• Gustatory.
• Resting vital signs (H.R, B.P, R. R) were checked before starting
the treatment.
• The environment around the patient was controlled with minimal
distractions as much as possible.
• Patient was made as comfortable as possible.
• Stimuli were organized in the above written manner.
• 45-50 min was given for coma stimulation program in which each
modality is given for 10 min. with adequate periods of rest.
• Each stimulus was presented for at least 2 min ago, interspaced
with rest periods and was not being repeated more often to
prevent habituation.
• Extra time was given to the patients to respond (because of slow
information processing).
• Verbal reinforcement of the responses were incorporated while
administration of auditory stimuli.
• Ongoing evaluation of stimuli had been done to which the patient
responds, as well as to these to which the patient does not
respond.
• Over stimulation was avoided indicated by flushing of the skin,
perspiration, agitation, eye closing, and sudden decrease in
arousal level, increase in muscle tone and prolonged increase in
respiratory rate.
• Participation of family member was facilitated.
Instrumentation
• Auditory: Through any electronic device.
• Visual: Familial pictures, torch.
• Olfactory: Coffee grinds, camphor, asafetida, perfume, shampoos.
• Tactile: Variety of texture of clothes.
• Gustatory: Cotton swabs dipped in sweet, salty and sour solutions.
• Neurological kit (Figures 2-5).
Statistical methodology
Research study design: This is a pre-test post-test experimental research design.
Study set up: The study is conducted in in-door patient department of Sri Aurobindo institute of medical sciences and post graduate institute, Indore (M.P).
Study duration: The duration of study was one year; September-2013 to August-2014.
Sampling: Convenient sampling technique is used for selection of desired samples according to inclusion-exclusion criterion.
Study tools:
• History.
• Examination.
• Glasgo Coma Scale (GCS).
The traumatic brain injury patients who admitted at study center and further met the inclusion criteria selected as subjects during specified schedule. A total of 15 traumatic head injury patients who were diagnosed as specified types of traumatic brain injury were conveniently selected from the in-door patient department at Sri Aurobindo institute of medical sciences and post graduate institute, Indore considered as subjects for the study. 15 TBI patients constituted study group were both, male and female. After necessary instructions and information about the study, the subjects attender had explained about the complete study procedure in his/ her own language and his/her willingness to participate in the study had recorded in a consent form dually signed by them. The study was approved by Institutional ethical Review Board (IRB).
The study group had analyzed the cases of specified type of traumatic brain injuries on Glasgo Coma Scale (GCS) in order to evaluate and compare the significance of coma stimulation at pre-intervention and after intervention by coma stimulation therapy to assess the effectiveness of therapy in cases of brain injuries such as diffuse axonal injury, frontal contusion, frontotemporal contusion, temporoparietal contusion, frontotemporoparietal contusion, parietal contusion and temporal contusion.
Statistical technique
The collected data were entered into the computer database and the responses of frequencies were calculated and analyzed by using various statistical tools. The responses of frequencies were calculated and analyzed by using the raw data of 15 subjects. Statistical software, SPSS version 17.0 was used for analysis. Prevalence of an outcome variable along with 95% confidence limits was calculated. Both, descriptive and inferential statistics were used to identify the effectiveness of coma stimulation therapy in traumatic head injury patients. Descriptive statistical analysis has used to depict the main features and characteristic of the collected data. Results on continuous measurements are presented on Mean ± SD (min-max) and results on categorical measurements are presented in numbers (%). A parametric test, unpaired t-test was used to identify the significance of difference in Glasgo Coma Scale (GCS) scores between observed different type of head injuries. Paired t-test was used to identify the significance of difference between pre-intervention and after intervention by coma stimulation therapy in study group.
One way Analysis of Variance (ANOVA) was used to identify the significance of mean differences in GCS scores among specified type of traumatic head injuries such as diffuse axonal injury, frontal contusion, frontotemporal contusion, temporoparietal contusion, frontotemporoparietal contusion, parietal contusion and Temporal contusion. The probability value, p>0.05 was considered as statistically insignificant but the probability value from p<0.05 to p<0.1 was considered as suggestively or poorly significant. The probability value from p<0.05 to p<0.01 was considered as statistically significant while from p<0.009 to p<0.001 was considered as statistically highly/strongly significant. Following are the notations used to present the significance of observed probability value.
Critical values and notations:
• ⊕ Insignificant/not significant (p-value: p>0.05).
• ^ Suggestively/poorly significant (p-value: 0.05<p<0.10).
• * Moderately significant/significant (p-value: 0.01<p<0.05).
• # Highly/strongly significant (p-value: 0.01<p<0.001).
Used formulae
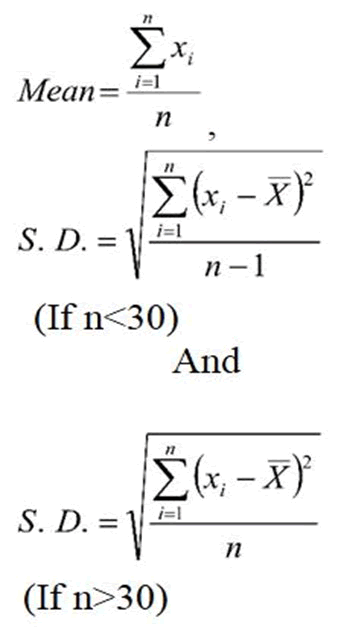
Where Ʃni=1 xi=Sum of all observations and
n=Number of subjects included for study according to inclusion criteria.
Ʃni=1(xi-x̄)2=Sum of squares of deviations from Mean.
The standard error of difference between two means is calculated by
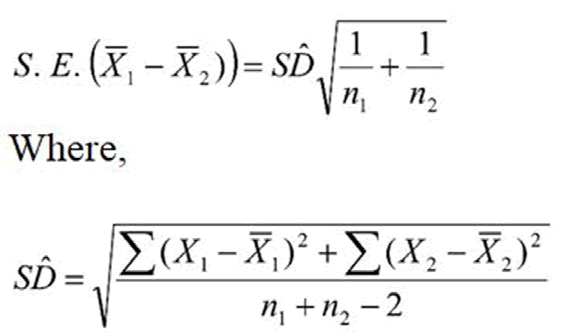
One-way ANOVA calculations now are common, for reference purposes the following steps describes how to calculate the various steps. The goal is to produce two variances (of treatments and error) and their ratio.
Step 1: Compute the correction for the mean (CM) by using the following formula-
CM=(Ʃji=1 Σkj=1 y ij )2/NTOTAL-CM=(Total of all observation)2/NTOTAL
Step 2: Compute the total Sum of Squares (SS)-
The total SS=SS (Total)=Sum of squares of all observations-CM
SS (Total)=Ʃji=1 Σkj=1 yij2/NTOTAL-CM
Step 3: Compute SST, the treatment sum of squares-First we compute the total (sum) for each treatment (groups) such as T1, T2, T3……… then SST=Ti2/ni-CM.
Step 4: Compute the error sum of squares (SSE)-SSE=SS (Total)-SST
Step 5: Compute mean square of treatments (MST), Mean Square of Error (MSE), and their ratio, F-MST=SST/k-1, MSE=SSE/N-k and F=MST/ MSE where N is the total number of observations, k is the number of groups (treatments) and n is size of groups.
The present study entitled “to find out the effectiveness of coma stimulation in specific types of traumatic brain injur” is carried out at indoor patient department of Sri Aurobindo institute of medical sciences and post graduate institute, Indore (M. P). A total of 15 cases of head injury treated as study elements that constituted study group (n=15) were conveniently selected as subjects for the present study. Out of 15 subjects, 12 (80.0%) were male while 3 (20.0%) were female. The age of all subjects were obtained in the ranges from 24 to 48 years. The spread of mean age in subjects with head injury were identified in the ranges of 35.07 ± 6.60 years. The following tables are showing the analyzed results with interpretations (Figure 6).
Table 1 highlighted the distribution of age for subjects with head injury. 46.7% cases of head injury were most common had belonged to age group of 32-40 years followed by 26.7% who had found within lower age group of 24-32 years. The age group of 40-48 years included 20.0% cases of head injury. Subjects, who had more than or equal to 48 years of age were very few was 6.7%.
| Age (year) | Frequency (N) | Percent (%) |
|---|---|---|
| 24-32 | 4 | 26.7 |
| 32-40 | 7 | 46.7 |
| 40-48 | 3 | 20 |
| ≥ 48 | 1 | 6.7 |
| Total | 15 | 100 |
| Mean ± SD | 35.07 ± 6.60 years | |
Table 1. Distribution of age of subjects.
Table 2 projected the group-wise gender distribution of head injury cases. 80.0% cases of head injury were obtained in male subjects. The cases of head injuries in female were only 20.0% (Figure 7).
| Age (year) | Frequency (N) | Percent (%) |
|---|---|---|
| Male | 12 | 80 |
| Female | 3 | 20 |
| Total | 15 | 100 |
Table 2. Distribution of gender of subjects.
It was easily seen in the Table 3 that the diffuse axonal injury was the most common head injury noted in one-third (33.3%) cases. The incidences of frontotemporal contusion and temporoparietal contusion were reported as the second most common head injuries recorded in each 2 (13.3%) case. The cases of frontotemporoparietal contusion, parietal contusion and temporal contusion head injuries were observed only in each 1 (6.7%) case (Figure 8).
| Diagnosis | Frequency (N) | Percent (%) |
|---|---|---|
| Diffuse axonal injury | 5 | 33.3 |
| Frontal contusion | 3 | 20 |
| Frontotemporal contusion | 2 | 13.3 |
| Temporoparietal contusion | 2 | 13.3 |
| Frontotemporoparietal contusion | 1 | 6.7 |
| Parietal contusion | 1 | 6.7 |
| Temporal contusion | 1 | 6.7 |
| Total | 15 | 100 |
Table 3. Diagnosis in head injury.
Table 4 highlighted that subjects with specific cases of head injury had significantly improved and differ Glasgo Coma Scale (GCS) scores that was measured after intervention by coma stimulation therapy as compared to the GCS scores at pre intervention stage. Henceforth, coma stimulation may be used to control the impairment of cognitive abilities in specified types of traumatic brain injury that impacted the effectiveness of coma stimulation therapy in traumatic brain injury. Research titled “To find out the effectiveness of coma stimulation in selected types of traumatic brain injury” (Figure 9).
| Parameter | Intervention stages | Glasgo Coma Scale (GCS) scores (N=15) | ||||
|---|---|---|---|---|---|---|
| Range (Mean ± SD) | 95% CI of the difference | t-value | LOS | |||
| LB | UB | |||||
| GCS score | Pre | 7.40 ± 0.74 | 2.55 | 5.85 | 5.46 | p<0.001 |
| Post | 11.60 ± 3.31 | |||||
| Mean difference | 4.2 | |||||
Table 4. Comparison between pre intervention and post intervention.
The mean difference is highly significant at the 0.001 level of significance degrees of freedom is 14; LOS-Level of Significance.
Table 5 highlighted that after administration of coma stimulation therapy at post-intervention stage the frontal contusion and frontotemporal contusion brain injuries had significantly improved and different but temporoparietal contusion was found to be poorly improved Glasgo Coma Scale (GCS) scores as compared to pre-intervention stage. The mean GCS score in subjects with Frontal contusion head injury at post-intervention stage was 14.67 ± 0.58 was higher and improved in comparison to score at pre-intervention stage was 7.67 ± 0.58 and this difference in mean GCS scores between pre and post intervention stages were strongly significant (p<0.008) that was confirmed on statistical ground (Figure 10).
| Head injury | Intervention stage | GCS score | MD | t-value | LOS |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Diffuse axonal | Pre | 7.00 ± 0.71 | 1.2 | 2.01 | p>0.05 |
| Post | 8.20 ± 1.92 | ||||
| Frontal contusion | Pre | 7.67 ± 0.58 | 7 | 12.12 | p<0.007 |
| Post | 14.67 ± 0.58 | ||||
| Frontotemporal contusion | Pre | 8.00 ± 0.0 | 5.5 | 11 | p<0.06 |
| Post | 13.50 ± 0.71 | ||||
| Temporoparietal contusion | Pre | 8.00 ± 0.0 | 6.5 | 1.37 | p<0.05 |
| Post | 14.50 ± 0.71 |
Table 5. Comparison in GCS scores for head injuries at pre intervention and post intervention.
In temporoparietal contusion head injury, at post-intervention stage the mean GCS score was (14.50 ± 0.71) higher and improved in comparison to score at pre-intervention stage (8.00 ± 0.0). The mean difference in GCS scores between pre and post intervention stages were statistically significant (p<0.05). The mean GCS score in subjects with frontotemporal contusion head injury at post-intervention stage was 13.50 ± 0.71 was higher and improved as compared to score at pre-intervention stage was 8.00 ± 0.0 and this difference in mean GCS scores between pre and post intervention stages were poorly significant (p<0.008) that was confirmed at 94.0% confidence interval on statistical ground.
In temporoparietal contusion head injury, at post-intervention stage the mean GCS score was (8.20 ± 1.92) little bit higher in comparison to score at pre-intervention stage (7.00 ± 0.71) but this mean difference in GCS scores between pre and post intervention stages wasn’t statistically significant (p>0.05). Moreover, it was noted that the subjects with frontal contusion head injury found with the highest improvement followed by temporoparietal contusion after administration of coma stimulation therapy which reflected significantly and better improved cognitive functions that impacted the effectiveness of coma stimulation therapy in traumatic brain injury.
Table 6 reported that without administration of coma stimulation therapy, the Glasgo Coma Scale (GCS) scores weren’t found to be depending on type of brain injury at pre-intervention stage. At pre intervention, all groups (type of brain injury) hadn’t significantly differed GCS scores, were found with approximately similar scores. One way Analysis of Variance (ANOVA) showed that there was not any statistical difference (p>0.05) detected in mean GCS scores among all selected head injuries. Since the test statistic (F=2.47) is lower than the critical value and therefore null hypothesis of equal population means was accepted. Further, it was conclude that there isn’t a significant (p>0.05) difference among the GCS score means for selected different head injuries that was confirmed statistically. Moreover, the mean GCS scores in diffuse axonal head injuries found with approximately similar GCS scores for frontotemporal contusion, temporoparietal contusion, frontotemporoparietal contusion, parietal contusion and temporal contusion head injuries.
| Type of brain injury | Group size (n) | Glasgo coma scale score | ||
|---|---|---|---|---|
| Mean ± SD | 95% CI for mean | |||
| LB | UB | |||
| Diffuse axonal injury | 5 | 7.00 ± 0.71 | 6.12 | 7.88 |
| Frontal contusion | 3 | 7.67 ± 0.58 | 6.23 | 9.1 |
| Frontotemporal contusion | 2 | 8.00 ± 0.00 | 8 | 8 |
| Temporoparietal contusion | 2 | 8.00 ± 0.00 | 8 | 8 |
| Frontotemporoparietal contusion | 1 | 7.00 ± 0.00 | - | - |
| Parietal contusion | 1 | 6.00 ± 0.00 | - | - |
| Temporal contusion | 1 | 8.00 ± 0.00 | - | - |
| Note: One way ANOVA (F=2.47; p>0.05; Insignificant). | ||||
Table 6. Comparison in Glasgo Coma Scale (GCS) scores at pre-intervention among specified types of head injury (groups).
It was easily seen in the Table 7 that the Glasgo Coma Scale (GCS) scores of subjects were significantly improved after administration of coma stimulation therapy and found to be depending on type of brain injury at post-intervention stage. At post-intervention stage all the groups with different type of brain injury had significantly differed GCS scores. The frontal contusion injury found with higher gain with a maximum of GCS score of 14.67 point followed by temporoparietal contusion (14.50) and parietal contusion (14.00) due to provided coma stimulation therapy. One way Analysis of Variance (ANOVA) showed that the GCS scores for different selected head injuries found to be strongly significant (p<0.002) mean differences that was concluded statistically among diffuse axonal, frontotemporal contusion, temporoparietal contusion, frontotemporoparietal contusion, parietal contusion and temporal contusion head injuries (Figure 11).
| Type of brain injury | Group size (n) | Glasgo coma scale score | ||
|---|---|---|---|---|
| Mean ± SD | 95% CI for Mean | |||
| LB | UB | |||
| Diffuse axonal injury | 5 | 8.20 ± 1.92 | 5.81 | 10.59 |
| Frontal contusion | 3 | 14.67 ± 0.58 | 13.23 | 16.1 |
| Frontotemporal contusion | 2 | 13.50 ± 0.71 | 7.15 | 19.85 |
| Temporoparietal contusion | 2 | 14.50 ± 0.71 | 8.15 | 20.85 |
| Frontotemporoparietal contusion | 1 | 7.00 ± 0.00 | - | - |
| Parietal contusion | 1 | 14.00 ± 0.00 | - | - |
| Temporal contusion | 1 | 12.00 ± 0.00 | - | - |
| Note: One way ANOVA (F=11.10; p<0.002; Highly significant) | ||||
Table 7. Comparison in Glasgo Coma Scale (GCS) scores at post-intervention among specified types of head injury (groups).
Moreover, the frontal contusion head injury subjects found with significantly and better improved cognitive functions measured with the highest GCS score followed by temporoparietal contusion and Parietal contusion. Finally, it was concluded that coma stimulation may be used to control the impairment of cognitive abilities in specified types of traumatic brain injury that impacted the effectiveness of coma stimulation therapy in traumatic brain injury.
Table 8 highlighted that subjects with diffuse axonal head injury had significantly different Glasgo Coma Scale (GCS) scores were significantly improved after administration of coma stimulation therapy but had approximately similar score at pre-intervention stage when compared with frontal contusion, frontotemporal contusion and temporoparietal contusion head injuries. At pre-intervention stage, the mean GCS score for subjects with diffuse axonal head injury was 7.00 ± 0.71 found statistically insignificant (p>0.05) when compared with GCS score in cases of frontal contusion (7.67 ± 0.58), frontotemporal contusion (8.00 ± 0.00) and temporoparietal contusion (8.00 ± 0.00) head injuries.
| Intervention | Type of head injury | GCS score | MD | t-value | LOS |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| At pre | Diffuse axonal | 7.00 ± 0.71 | 0.67 | 1.37 | p>0.05 |
| Frontal contusion | 7.67 ± 0.58 | ||||
| At post | Diffuse axonal | 8.20 ± 1.92 | 6.47 | 5.52 | p<0.001 |
| Frontal contusion | 14.67 ± 0.58 | ||||
| At pre | Diffuse axonal | 7.00 ± 0.71 | 1 | 1.89 | p>0.05 |
| Frontotemporal contusion | 8.00 ± 0.00 | ||||
| At post | Diffuse axonal | 8.20 ± 1.92 | 5.3 | 3.62 | p<0.02 |
| Frontotemporal contusion | 13.50 ± 0.71 | ||||
| At pre | Diffuse axonal | 7.00 ± 0.71 | 1 | 1.89 | p>0.05 |
| Temporoparietal contusion | 8.00 ± 0.00 | ||||
| At post | Diffuse axonal | 8.20 ± 1.92 | 6.3 | 4.31 | p<0.008 |
| Temporoparietal contusion | 14.50 ± 0.71 |
Table 8. Comparison in GCS scores between diffuse axonal, frontal contusion, frontotemporal contusion and temporoparietal contusion at pre and post interventions.
The coma stimulation therapy found effective as the mean GCS scores for subjects with frontal contusion head injury was significantly maximum at post intervention stage were 14.67 ± 0.58 points as compared to mean GCS score for subjects with diffuse axonal head injury was 8.20 ± 1.92 points. This difference in mean GCS score (6.47) was strongly significant (p<0.001) that was concluded statistically. After administration of coma stimulation therapy, the mean GCS scores for subjects with Frontotemporal contusion head injury was higher were 13.50 ± 0.71 points as compared to mean GCS score for subjects with diffuse axonal head injury was 8.20 ± 1.92 points and the observed difference in mean GCS score was 5.30 points were measured significant (p<0.02) confirmed on statistical ground. The mean GCS scores in temporoparietal contusion head injury was significantly higher at post intervention stage were 14.50 ± 0.71 points as compared to mean GCS score in diffuse axonal head injury was 8.20 ± 1.92 points. This difference in mean GCS score (6.30) was strongly significant (p<0.008) confirmed statistically.
Moreover, it was noted that the frontal contusion head injury found with the highest score measured on Glasgo Coma scale after administration of coma stimulation therapy which reflected significantly and better improved cognitive functions in frontal contusion head injury case followed by Temporoparietal contusion and parietal contusion.
Table 9 highlighted that traumatic brain injury had insignificant Glasgow Coma Scale (GCS) scores at pre-intervention stage between male and female while the GCS scores were improved after administration of coma stimulation therapy at post-intervention stage but measured not significant between male and female due to equal response to therapy by both the genders. When the mean GCS score for male subjects with head injury at pre-intervention stage was (7.42 ± 0.79) compared with female subjects (7.33 ± 0.58) was measured just similar and therefore statistically insignificant (p>0.05).
| Intervention stage | Gender | GCS score | MD | t-value | LOS |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| At pre | Male | 7.42 ± 0.79 | 0.09 | 0.17 | p>0.05 |
| Female | 7.33 ± 0.58 | ||||
| At post | Male | 12.17 ± 3.22 | 2.84 | 1.37 | p>0.05 |
| Female | 9.33 ± 3.22 |
Table 9. Comparison in male female at pre intervention and post intervention.
The coma stimulation therapy found effective as the GCS scores for male subjects were improved and higher at post intervention stage were 12.17 ± 3.22 points as compared to mean GCS scores for female were 9.33 ± 3.22 points but the difference in mean GCS score for genders was 2.84 that was confirmed statistically not significant (p>0.05). Moreover, it was noted that administration of coma stimulation therapy in cases of head injuries responded equally in genders, in male as well as in female due to insignificant mean differences in GCS scores which reflected that male and female both were reported with improved cognitive functions. No gender determination was noted regarding administration of coma stimulation therapy.
It was concluded that coma stimulation may be used to control the impairment of cognitive abilities for both male and female in specified types of traumatic brain injury that impacted the effectiveness of coma stimulation therapy in traumatic brain injury. Furthermore, it was noted in cases of head injury that administration of coma stimulation therapy was effective that significantly improved cognitive functions in selected traumatic brain injuries. Lastly, all the depicted tables and above stated all inferences indicated rejection of null hypothesis and acceptance of alternative hypothesis i.e. Coma stimulation programme showed significantly better improvement in Frontal contusion injury found with higher gain followed by Temporoparietal contusion and Parietal contusion impacted the achievement of the entire selected objectives followed with fulfillment of the aim and the objectives of the proposed research titled “To find out the effectiveness of coma stimulation in selected types of traumatic brain injuries”.
Compairision between pre intervention and post intervention Glasgow coma scale score in all types of traumatic brain injury. The mean GCS scores of specified cases in head injury after treatment by coma stimulation therapy was 11.60 ± 3.31 points was higher as compared to initial stage was 7.40 ± 0.74 scores noted at pre intervention stage. This difference in mean GCS scores between pre and post intervention was strongly significant (p<0.001) confirmed statistically. Moreover, it was concreted statistically that specified cases of head injury found with significantly improved cognitive functions reflected by better increased GCS s core.
Comparison in Glasgo Coma Scale (GCS) scores at preintervention among specified types of head injury (groups): One way Analysis of Variance (ANOVA) showed that there was not any statistical difference (p>0.05) detected in mean GCS scores among all selected head injuries. Since the test statistic (F=2.47) is lower than the critical value and therefore null hypothesis of equal population means was accepted. Further, it was conclude that there isn’t a significant (p>0.05) difference among the GCS score means for selected different head injuries that was confirmed statistically. Moreover, the mean GCS scores in diffuse axonal head injuries found with approximately similar GCS scores for frontotemporal contusion, temporoparietal contusion, frontotemporoparietal contusion, parietal contusion and temporal contusion head injuries.
Comparison in Glasgo Coma Scale (GCS) scores at postintervention among specified types of head injury (groups): One way Analysis of Variance (ANOVA) showed that the GCS scores for different selected head injuries found to be strongly significant (p<0.002) mean differences that was concluded statistically among diffuse axonal, frontotemporal contusion, temporoparietal contusion, frontotemporoparietal contusion, parietal contusion and temporal contusion head injuries. Moreover, the Frontal contusion head injury subjects found with significantly and better improved cognitive functions measured with the highest GCS score followed by temporoparietal contusion and parietal contusion. Comparison in GCS scores for head injuries at pre intervention and post intervention: The mean GCS score in subjects with frontal contusion head injury at post-intervention stage was 14.67 ± 0.58 was higher and improved in comparison to score at pre-intervention stage was 7.67 ± 0.58 and this difference in mean GCS scores between pre and post intervention stages were strongly significant (p<0.008) that was confirmed on statistical ground. In temporoparietal contusion head injury, at post-intervention stage the mean GCS score was (14.50 ± 0.71) higher and improved in comparison to score at pre-intervention stage (8.00 ± 0.0). The mean difference in GCS scores between pre and post intervention stages were statistically significant (p<0.05).
The mean GCS score in subjects with Frontotemporal contusion head injury at post-intervention stage was 13.50 ± 0.71 was higher and improved as compared to score at pre-intervention stage was 8.00 ± 0.0 and this difference in mean GCS scores between pre and post intervention stages were poorly significant (p<0.008) that was confirmed at 94.0% confidence interval on statistical ground. In Diffuse axonal injury, at post-intervention stage the mean GCS score was (8.20 ± 1.92) little bit higher in comparison to score at pre-intervention stage (7.00 ± 0.71) but This mean difference in GCS scores between pre and post intervention stages wasn’t statistically significant (p>0.05).
Moreover, it was noted that the subjects with Frontal contusion head injury found with the highest improvement followed by Temporoparietal contusion after administration of coma stimulation therapy which reflected significantly and better improved cognitive functions that impacted the effectiveness of coma stimulation therapy in traumatic brain injury. Comparison in GCS scores between diffuse axonal, frontal contusion, frontotemporal contusion and temporoparietal contusion at pre and post interventions: At pre-intervention stage, the mean GCS score for subjects with diffuse axonal head injury was 7.00 ± 0.71 found statistically insignificant (p>0.05) when compared with GCS score in cases of frontal contusion (7.67 ± 0.58), frontotemporal contusion (8.00 ± 0.00) and temporoparietal contusion (8.00 ± 0.00) head injuries.
The coma stimulation therapy found effective as the mean GCS scores for subjects with frontal contusion head injury was significantly maximum at post intervention stage were 14.67 ± 0.58 points as compared to mean GCS score for subjects with diffuse axonal head injury was 8.20 ± 1.92 points. This difference in mean GCS score (6.47) was strongly significant (p<0.001) that was concluded statistically. After administration of coma stimulation therapy, the mean GCS scores for subjects with Frontotemporal contusion head injury was higher were 13.50 ± 0.71 points as compared to mean GCS score for subjects with diffuse axonal head injury was 8.20 ± 1.92 points and the observed difference in mean GCS score was 5.30 points were measured significant (p<0.02) confirmed on statistical ground.
The mean GCS scores in temporoparietal contusion head injury was significantly higher at post intervention stage were 14.50 ± 0.71 points as compared to mean GCS score in diffuse axonal head injury was 8.20 ± 1.92 points. This difference in mean GCS score (6.30) was strongly significant (p<0.008) confirmed statistically. Moreover, it was noted that the Frontal contusion head injury found with the highest score measured on Glasgo coma scale after administration of coma stimulation therapy which reflected significantly and better improved cognitive functions in Frontal contusion head injury case followed by temporoparietal contusion and Parietal contusion.
Unconsciousness is a common complication after sever brain injury. Recovery from a post injury unconscious state usually depends on injury severity and other complex physiological responses to the injury. Most of the studies in traumatic brain injury are done to find out efficacy of coma stimulation program in traumatic brain injury. The objective of the present study is to study the effect of coma stimulation program in cases of frontal, temporal, parietal, frontotemporal, frontotemporoparietal, temporoparietal, and diffuse axonal injury by comparing GCS score pre and post intervention. Results from this study suggest that providing repetitive coma stimulation program for 4 weeks can enhance consciousness recovery in traumatic brain injured patients. Significant improvement in response to coma stimulation program as measured by Glasgow coma scale could be seen post intervention in all included cases except diffuse axonal in jury.
Brain damage following traumatic injury is a result of direct and indirect mechanism. These secondary mechanisms involve the initiation of an acute inflammatory response including breakdown of blood brain barrier, edema formation and swelling, infiltration of peripheral blood cells and activation of resident immunocompotent cells, as well as the intrathecal release of numerous immune mediators such as interleukins and chemotactic factors. the possible harmful/beneficial sequalae of post-trauamtic inflammation in the CNS occur by three mediators of inflammation in the brain, Tumor- Necrosis Factor-alpha (TNF-alpha), Interleukin-6 (IL-6), and transforming growth factor beta (TGF-beta), while the former two may act as important mediators for the initiation and support of post-traumatic inflammation, thus causing additional cell death and neurologic dysfunction they also enhances reparative processes. TGF-beta, on the other hand is potent anti-inflammatory agent, which may also have some deleterious long term effects in the injured brain.
Patient in the unconsciousness experience sensory deprivation because their ability to respond internal and external stimuli is altered. Because of this alteration, the threshold of the activation of the reticular activating system may increase. The practical implication of sensory deprivation is that controlled stimulation (consisting auditory, gustatory, olfactory, tactile, kinesthetic, visual modes) may meet higher threshold of reticular neurons and increase cortical activity or that the undamaged axons may actually send out collateral connections, called collateral spouting, which assist in reorganizing the brain's activity. On the basis of animal model sensory stimulation of sufficient frequency, intensity, and duration was shown to arouse the brain by improving neuronal organization, increased number of dendritic branching, and increased number of dendritic spines, stimulating the reticular activating system and increasing the level of cognitive function after brain injury.
Results showed that after administration of coma stimulation program at post intervention stage the frontal contusion, frontotemporal contusion and temporoparietal contusion had significantly improved but diffuse axonal injury found to be poorly improved Glasgow coma scale score as compare to pre intervention stage. A conscious state depends as intact cerebral hemispheres, interacting with the ascending reticular activating system in the brain stem, midbrain, hypothalamus and thalamus. Lesions affecting the cerebral hemisphere, or directly affecting the reticular activating system cause impairment of conscious level. The ascending reticular pathway is also called reticular activating system. It is a complex polysynaptic pathway which extends from the lower pons to the level of thalamus. It constitutes the cell bodies and fibers mainly of cholinergic system. RAS throughout its course receives afferent collateral from long somatic sensory pathways, the trigeminal, olfactory auditory and visual pathway and visceral pathways. A single peripheral stimulation gives rise impulses which ascend to cerebral cortex. Activity in the RAS produces the conscious state and is responsible for maintaining a state of wakefulness or alertness. This makes perception possible deactivation of RAS produces sleep. Destruction of RAS produces coma or unconsciousness.
Contusion is a focal damage to brain these may occur under or opposite the site of impact, but most commonly involve frontal and temporal lobes. Contusions in frontal and temporal lobes often lead to bleeding into brain substance usually associated with an overlying subdural hematoma. Injuries such as intracerebral hematoma and cerbral contusion results in blood intermixed with brain. In cases of contusion, the primary pathology includes primary damage to neuronal cells. In both instances the presence of blood within brain tissue appears to have significant toxic effects which can produce profound secondary insult to the injured tissue. In the early phase after contusional trauma, inflammation is mainly intravascular and dominated by polymorph nuclear cells. The brain swelling present. The delayed phase correlating with a parenchymal inflammation. The inflammatory cells may produce several potentially harmful effects, such as acute cellular degeneration; they may also lead to degenerative long term effects. Influence of inflammatory mechanism producing secondary deterioration.
Discharging cortical lesions due to torn bridges veins or hemorrhages, expands inside the brain, thereby increasing intracranial pressure and leading to brain deformation, which is compensated by efflux of cerebrospinal fluid towards the spinal axis. This ultimately led to compression of the venous system. In this process, there is an initial small change in intracranial pressure. Later, compensatory mechanism decreases ultimately leading to exponential increment in intracranial pressure, causing compressive forces on the reticular activating system and reticular formations. Thus there is decrease in the level of consciousness and an exponential increase in ICP decreases cerebral perfusion which will lead to global cerebral ischemia.
Measures taken to reduce ICP reduce compressive forces on the RAS and neural recovery in the cortex initiates in course of recovery in cerebral contusions. Neuronal changes in the neuronal recovery include axonal swelling and degeneration and loss of synapses, the sites of communication between neuron, this is followed by axonal sprouting and alteration in synaptic markers in recovery. Axonal sprouting occurs in areas of focal injury, indicating recovery after TBI. Coma stimulation may meet higher threshold of reticular neurons and increase cortical activity or that undamaged axons may actually send out collateral spouting which assist in reorganizing brain's activity.
After brain contusion, neural plasticity mechanism required for recovery. Post traumatic plasticity involves aspects of neurogenesis, angiogenesis, axonal spouting and synaptic formation and remodelling. Scheff and Semchenko, et al., both showed that synapse number after TBI begins to recover at approximately 10-14 days post injury, and is nearly completely recovered at 1 month post injury. Sensory stimulation of sufficient frequency, intensity and duration was shown to arouse the brain by improving neuronal organization, increased dendritic branching and increased numbers of dendritic spines, stimulating RAS, and increasing level of cognitive function. Environment plays a crucial role in promoting neurological recovery of function. As the brain adjusts, its use of newly acquired function drives plastic changes, while disuse of other area diminishes changes.
Sensory stimulation program allow the patients to follow a structured stimulation, optimizing their aptitude to react and to respond to their environment. These would constitute enriched environment that would potentially have an impact on the structure and the function of the brain and hence on the brain plasticity. Brain tissue has capacity to adapt and transform according to environment to restructure connection between neuron in order to develop networks, which optimize the circulation of information provided by the environment. The adult nervous system would be vulnerable and hence sensitive to modification and adaptation. Sensory stimulation program can be considered as enriched environment. The principle of enriched environment was used by researcher studying animal behavior. These experiment consisted is using ample animal boxes containing various objects stimulating curiosity and manipulation. It is an approach can change the brain on a neuroanatomical and biochemical level.
On the other hand in the in the diffuse atonal injury, axonal injury occurs from inertial forces applied to head is associated with prolonged unconsciousness and poor outcome. The susceptibility of axon to mechanical injury appears to be due to both their viscoelastic properties and their highly organized structure in white mater tracts. Although axons are supple under normal conditions, they become brittle when exposed to rapid deformations associated with brain trauma. Accordingly rapid stretch of axons cytoskeleton, resulting in a loss of elasticity and impairment of axoplasmic transport, subsequent swelling of the axon occur in discrete bulb formation or in elongated viscosities that accumulate organelles. Calcium entry into damaged axons is thought to initiate further damage by the activation of proteases and induction of mitochondria swelling and dysfunction. Ultimately, swollen axons may become disconnected and contribute to additional neuropath logical changes in brain tissues. Widespread damage to the white matter will produce a recovery that is characteristically slow and incomplete. Coma stimulation in diffuse axonal injury blocked due to impaired axoplasmic transport and then its effect is not show on post intervention GCS score of subjects with diffuse axo nal injury.
Unconscious patients after brain injury may survive for days or months and often experience decreased quality of life due to state of unconsciousness. The impact of injuries play major role in recovery process by coma stimulation program. The result from this study suggested that patients of contusion brain injury showed better improvement in comparison of diffuse axonal injury, thereby indicating pathophysiology of recovery induced by coma stimulation program. Hence the Null hypothesis Coma stimulation program may not beneficial for the patients of all varieties of traumatic brain injury is accepted.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
International Journal of Neurorehabilitation received 1078 citations as per Google Scholar report