Research - (2022) Volume 13, Issue 4
Received: 31-Mar-2022, Manuscript No. CSJ-22-65462;
Editor assigned: 03-Apr-2022, Pre QC No. P-65462;
Reviewed: 17-Apr-2022, QC No. Q-65462;
Revised: 22-Apr-2022, Manuscript No. R-65462;
Published:
29-Apr-2022
, DOI: 10.37421/2150-3494.2022.13.285
Citation: Eliashvili, Levani, Lamzira Pharulava and Vakhtang
Ugrekhelidze. “Determination of Atomic Fractions of Oxygen Isotopes in
Isotopically Modified Nitrogen Oxide, Water, and Molecular Oxygen.” Chem Sci
J 13 (2022): 285.
Copyright: © 2022 Eliashvili L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The atomic fractions of the isotopes of oxygen are determined in nitrogen oxide and water by conversion into molecular oxygen. Determination of the isotopes of oxygen in molecular oxygen is made utilizing intensities of ionic currents of various mass numbers and the atomic fraction of oxygen isotopes, which are calculated by different formulas, thus verifying the possible background superimposition of any molecular line and that significantly increases the reliability and accuracy of the method.
Atomic fraction • Oxygen • Nitrogen • Water • Nitrogen oxide • Molecular oxygen • Molecular ions • Mass spectrometer • Monoisotopic • Isotopically modified
The mass-spectrometric method of isotopic analysis is mostly used for the determination of the atomic fraction of isotopes of an element in substances. The atomic fraction of elements of gaseous substances is determined by mass spectrometers, where ionization is achieved by the electronic bombardment method. The mass-spectrometric method directly records the intensity of ions of substances and does not depend on any physical and chemical properties of the substance. It is distinguished by high informative value and accuracy
Despite the abovementioned, the mass-spectrometric method is characterized by certain complications. Some compounds labeled with the stable isotopes oxygen-17 and oxygen-18 is widely used in medical diagnostics and their field of application is gradually expanding. The use of compounds labeled with stable oxygen isotopes makes it possible to solve problems that could not be solved without their use. To determine the atomic fraction of oxygen in water, it is converted into molecular oxygen by the socalled Urey method.
Several papers are devoted to the determination of the atomic fraction of oxygen isotopes in molecular oxygen. The electronic bombardment of molecular oxygen forms the isobaric monoisotopic ions 17O17O+ and 16O18O+, and the mass spectrometer with high separation capacity, on which it is possible to record them separately, is not available. Therefore, some authors do not take into account the ions 17O17O+ and the calculation is made by simplified formula. When the content of isotopes oxygen-17 is small, the inaccuracy obtained by neglecting these ions is commensurate with the measuring inaccuracy. However, in recent periods an interest in isotopes of oxygen-17 has increased and such products of molecular oxygen have been produced, in which the content of this isotope even achieves 99%.
The proposed method takes into account the superposition of monoisotopic ions, also it is possible to calculate the isotope oxygen-17 utilizing molecular ions with various mass numbers m/z, which allows recording the inaccuracy caused by background superposition on some mass peak, i.e., the method is self-checking. The sampling and the process of conversion into molecular oxygen should take into consideration that the content of isotopes of molecular oxygen adequately reflects the content of atomic fractions of oxygen isotopes in nitrogen oxide and isotopically modified water. The sampling and the process of conversion should be conducted by high standard, i.e., the requirements for vacuum conditions are to be increased and at the same time, the surfaces of sampling and conversion systems must be meticulously treated. Possibly, it will become necessary to “wash” the surface by the analyzed sample, if two subsequent analyses of oxygen isotopes have a big difference. At electronic bombardment of molecular oxygen, the following monoisotopic forms arise 16O16O+, 16O17O+, 16O18O+, 17O17O+, 17O18O+, 18O18O+, i.e., the ions whose mass numbers m/z are respectively 32, 33, 34, 35, 36. Any three combinations of intensities of these ion peaks are sufficient for the determination of atomic fractions of oxygen isotopes.
Nitrogen oxide is used for the separation of the isotopes of oxygen and nitrogen in the separation plant by the method of low-temperature distillation. Isotopically modified water and molecular oxygen are obtained from isotopically modified nitrogen oxide for further processing. To control the operation mode of the separation plant and to certify the ready product in nitrogen oxide, as well as in isotopically modified water and molecular oxygen, is proposed method is self-verifying and allows the detection of background mass distortion of any mass line, resulting in a highly accurate and reliable method by which any three molecular lines can determine the atomic fraction of oxygen isotopes and test the results. The method is used for the certification of molecular oxygen and water at the National High Technology Center of Georgia (formerly the Institute for Stable Isotopes).
The measurements of atomic fractions of the isotope’s oxygen-16, oxygen-17, and oxygen-18 in nitrogen oxide, water, and molecular oxygen in each case were conducted following the requirements of the standard sample. The method is self-checking and it is successfully used for testing the ready product. By the method, a determination of the atomic fraction of oxygen isotopes in nitrogen oxide and water and molecular nitrogen, obtained from it, has been conducted. The obtained results agree with each other with high accuracy. In addition, by verifying of dilution method, the conducted experiments also have demonstrated the agreement of the calculated and measured values.
Experimental
The proposed methods of determination of atomic fractions of nitrogen and oxygen isotopes in nitrogen oxide do not ensure high reliability and accuracy. Due to its high reactive capacity nitrogen oxide quickly reacts with oxygen adsorbed on a surface and forms nitrogen dioxide [1], after the secondary process takes place in the ionization chamber with high probability [1-3]. It easily dissociates under electronic bombardment and nitrogen oxide arises again, as a result, the content of oxygen isotopes in nitrogen oxide is changed by oxygen absorbed on a surface. The degree of dilution depends on the degree of outgassing of surfaces of the mass-spectrometer, the partial pressure of nitrogen oxide, and the difference of values of atomic fractions of oxygen isotopes in comparison with the natural oxide. Except for the abovementioned, nitrogen oxide is characterized by high adsorption properties, i.e. by the so-called memory effect. By prolonged high-temperature outgassing of the mass spectrometer and repeated “washing” of the analyzed sample the effect can be minimized. It should be noted that nitrogen oxide obtained in separation plants is not isotopically balanced, that infracts the principle of proportionality of isotope concentrations, so the conduction of isotopic analysis becomes impossible. In the isotopically balanced nitrogen oxide, even after overcoming these complications, on isotopic mass peaks with the mass numbers m/z=31, 32 we have a superposition of monoisotopic ions, the ions 14N17O+and 15N16O+ are recorded as the ionic current with one mass number m/z=31, and the ionic currents 14N18O+ and 15N17O+ as the ionic current with the mass number m/z=32. In such a case it is possible to conduct the isotopic analysis for nitrogen and as well for oxygen.
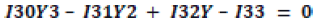 (1)
(1)
By this equation the ratio
Y=15N/14N (2)
of nitrogen isotopes is calculated, where I30, I31, I32, I33 are respectively the intensities of ions with mass numbers m/z=30, 31, 32, 33.
The atomic fraction of the isotope nitrogen-15 is calculated by the formula:
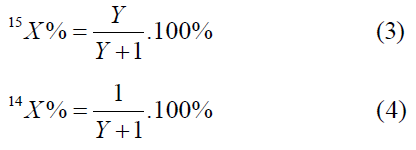
The atomic fraction of the isotopes of oxygen is calculated by the formulas:
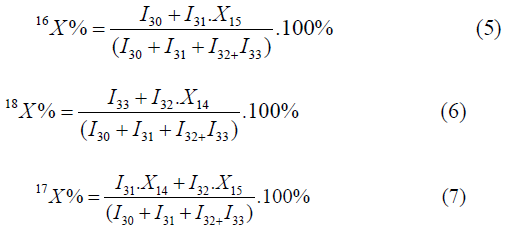
In this way, it is possible to determine the values of all three oxygen isotopes. In the methods, proposed in the literature (4,5) the isotope oxygen-17 is not taken into consideration, and in separation plants in parallel the content of oxygen-17 increases; it is necessary to determine all three isotopes of oxygen simultaneously. Based on the above, to achieve reliability and high accuracy it is necessary to decompose nitrogen oxide into molecular oxygen and molecular nitrogen. The mass-spectrometric method of determination of atomic fractions of oxygen isotopes directly in water is not known that is conditioned by the following factors; it has sharply expressed adsorption properties that cause the so-called memory effect; water is hardly desorbed from surfaces even during prolonged outgassing the conditions of high vacuum at 2000 ºC temperature, as a result, it is impossible to eliminate the background peak. In addition, as a result of fragmentation by hydrogen abstraction, a formation of isobaric fragmented ions and their superposition on the molecular ions take place.
The paper proposes a method that allows determining atomic fractions of oxygen isotopes by the values of ionic currents of various ratios m/z.
The atomic fractions of the oxygen isotopes using the mass peaks m/ z=32, 33, 34 are calculated by the following formulas:
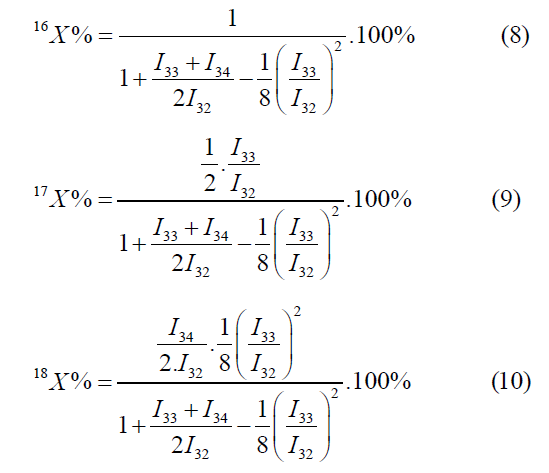
The calculation by the mass peaks m/z=32, 33, 35 is made by the foll
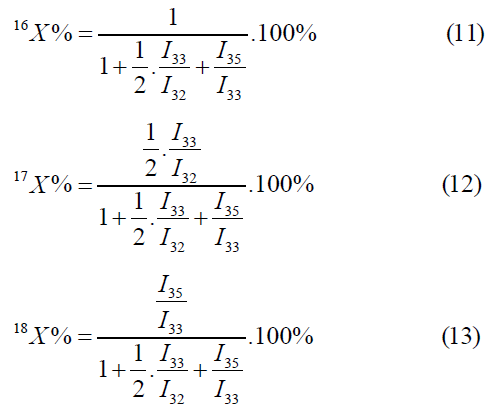
The atomic fraction of the isotopes of oxygen is calculated by the mass peaks m/z=32, 33, 36.
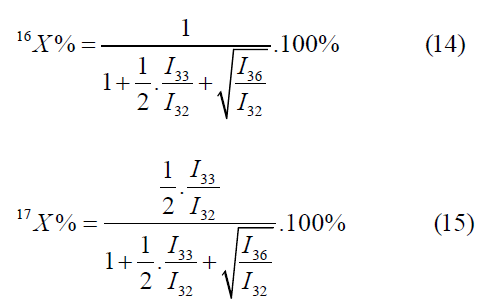
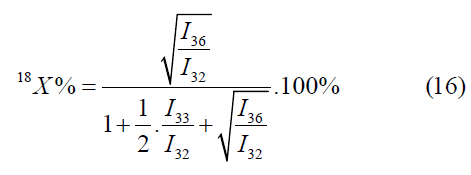
The formulas for the mass peaks m/z=32, 34, 36 are as follows:
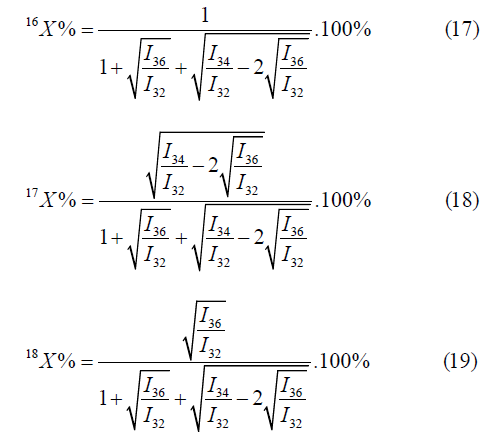
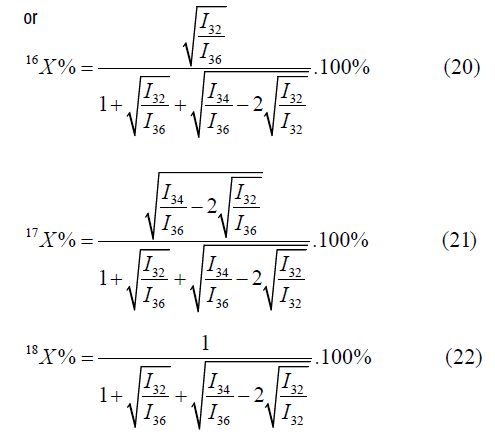
The calculation by the mass peaks m/z=32, 35, 36 is made by the following formulas:
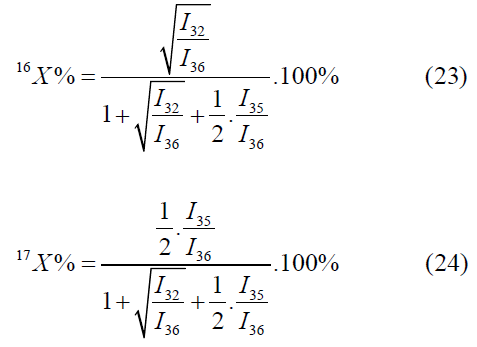
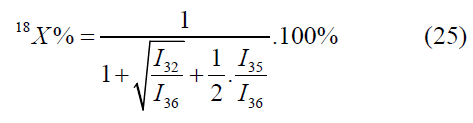
The calculation by the mass peaks m/z=33, 35, 36 is made by the following formulas:
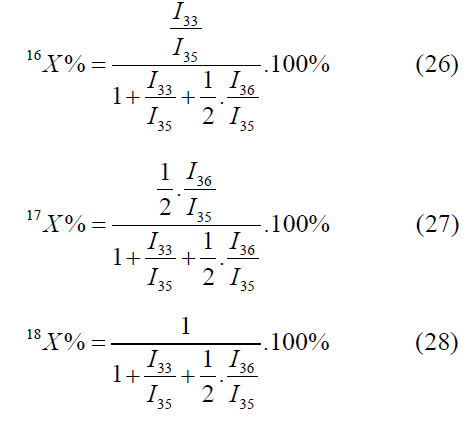
The atomic fraction of oxygen isotopes may be calculated by the following mass peaks m/z=34, 35, 36;
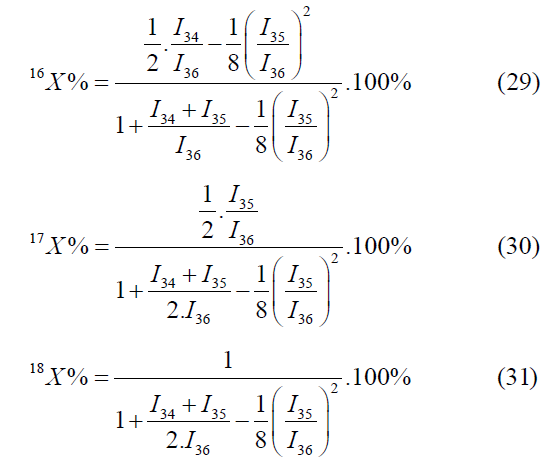
The formulas for calculation by the mass peaks m/z=32, 34, 35 are as follows:
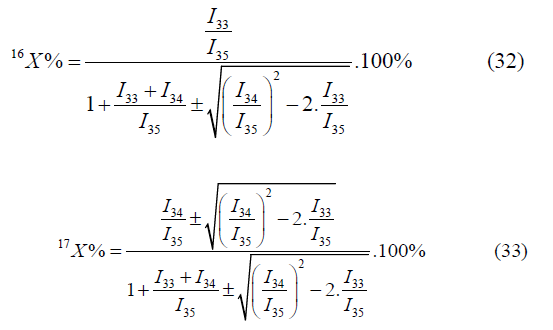
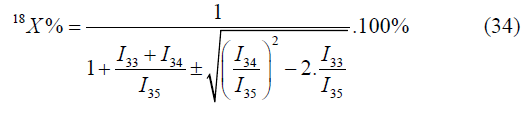
In the given case one value will be selected out of two values by means of values of atomic fractions, calculated by other mass peaks [4-6].
With such an approach to isotopic analysis, it is possible to make the method self-checking. By analyzing values obtained by different formulas the analyst can easily determine the presence of background superposition on some mass peaks that may be caused by impurities in the sample, or by background superposition presented in the mass-spectrometer. Such a possibility of analysis increases the accuracy and reliability of the measurement.
The superposition on the mass m/z=32 may be checked by the formula:
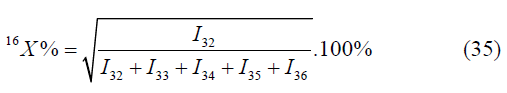
A deviation from atomic fractions of the isotope oxygen-16, calculated by another formula, obviously points out the superposition on the mass peak m/ z=32. Similarly, the superposition on the mass peak m/z may be checked by the formula:
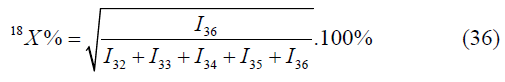
Also, the true values may be checked by the formula, calculated by the mass peaks m/z=I32, I33, I34, I35, I36 (Table 1);
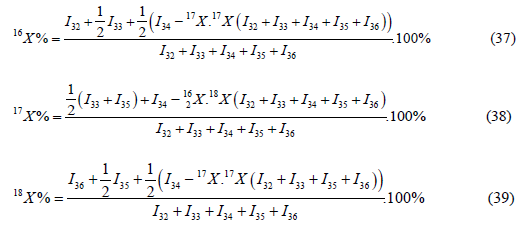
| S. No | The Atomic Fractions of Oxygen Isotopes, Calculated by the Mass Peaks | 32, 33, 34 | 32,33, 35 | 32, 33, 36 | 32, 34, 36 | 32, 35, 36 | 33, 35, 36 | 33, 34, 35 | 34, 35, 36 | √ | ∑ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16O | 24,77 | 24,77 | 24,77 | 24,77 | 24,75 | 24,73 | 24,77 | 23,87 | 24,77 | 24,77 |
| 17O | 70,78 | 70,78 | 70,78 | 70,78 | 70,81 | 70,82 | 70,78 | 71,64 | - | 70,78 | |
| 18O | 4,46 | 4,45 | 4,45 | 4,45 | 4,44 | 4,45 | 4,45 | 4,50 | 4,45 | 4,45 | |
| 2 | 16O | 36,29 | 36,29 | 36,30 | 36,29 | 36,29 | 36,26 | 36,29 | 36,29 | 36,29 | 36,29 |
| 17O | 34,40 | 34,40 | 34,41 | 34,43 | 34,43 | 34,44 | 34,40 | 34,43 | - | 34,42 | |
| 18O | 29,31 | 29,31 | 29,29 | 29,28 | 29,28 | 29,29 | 29,31 | 29,28 | 29,29 | 29,29 | |
| 3 | 16O | 49,07 | 49,07 | 49,06 | 49,07 | 49,23 | 49,41 | 49,07 | 53,91 | 49,07 | 49,07 |
| 17O | 49,31 | 49,31 | 49,31 | 49,30 | 49,13 | 48,97 | 49,32 | 44,61 | - | 49,31 | |
| 18O | 1,62 | 1,62 | 1,63 | 1,63 | 1,63 | 1,63 | 1,62 | 1,48 | 1,63 | 1,62 | |
| 4 | 16O | 0,43 | 0,37 | 0,39 | - | 0,39 | 0,37 | 0,37 | 0,36 | 0,39 | 0,36 |
| 17O | 0,33 | 0,29 | 0,30 | - | 0,32 | 0,32 | 0,33 | 0,32 | - | 0,32 | |
| 18O | 99,24 | 99,35 | 99,30 | - | 99,28 | 99,31 | 99,30 | 99,32 | 99,32 | 99,32 | |
| 5 | 16O | 3,66 | 3,66 | 3,66 | 3,66 | 3,65 | 3,64 | 3,66 | - | 3,66 | 3,66 |
| 17O | 91,16 | 91,18 | 91,19 | 91,19 | 91,21 | 91,22 | 91,16 | - | - | 91,18 | |
| 18O | 5,18 | 5,16 | 5,15 | 5,15 | 5,14 | 5,14 | 5,16 | - | 5,15 | 5,16 | |
| 6 | 16O | 0,40 | 0,39 | 0,39 | - | 0,39 | 0,39 | 0,39 | 0,38 | 0,39 | 0,38 |
| 17O | 0,58 | 0,57 | 0,57 | - | 0,57 | 0,57 | 0,58 | 0,58 | - | 0,57 | |
| 18O | 91,02 | 99,04 | 99,04 | - | 99,04 | 99,04 | 99,03 | 99,04 | 99,04 | 99,04 | |
| 7 | 16O | 83,64 | 83,62 | 83,65 | 83,54 | 83,59 | 83,49 | 83,58 | 83,61 | 83,64 | 83,64 |
| 17O | 10,41 | 10,40 | 10,41 | 10,53 | 10,47 | 10,54 | 10,45 | 10,46 | - | 10,41 | |
| 18O | 5,95 | 5,95 | 5,94 | 5,93 | 5,93 | 5,97 | 5,97 | 5,93 | 5,94 | 5,95 | |
| 8 | 16O | 78,78 | 78,77 | 78,78 | 78,79 | 78,60 | 78,40 | 78,92 | 77,47 | 78,78 | 78,78 |
| 17O | 20,22 | 20,22 | 20,22 | 20,21 | 20,41 | 20,59 | 20,06 | 21,48 | - | 20,22 | |
| 18O | 1,00 | 1,01 | 1,00 | 1,00 | 1,01 | 1,01 | 1,01 | 1,05 | 1,00 | 1,00 | |
| 9 | 16O | 29,84 | 29,81 | 29,82 | 29,84 | 29,81 | 29,78 | - | 29,71 | 29,82 | 29,81 |
| 17O | 40,58 | 40,53 | 40,55 | 40,52 | 40,58 | 40,59 | - | 40,63 | - | 40,53 | |
| 18O | 29,58 | 29,65 | 29,63 | 29,64 | 29,61 | 29,62 | - | 29,63 | 29,63 | 29,63 | |
| 10 | 16O | 33,20 | 33,21 | 33,21 | 33,21 | 33,21 | 33,20 | 33,20 | 33,18 | 33,21 | 33,21 |
| 17O | 59,17 | 59,18 | 59,19 | 59,19 | 59,19 | 59,20 | 59,20 | 59,22 | - | 59,19 | |
| 18O | 7,63 | 7,60 | 7,60 | 7,60 | 7,60 | 7,60 | 7,60 | 7,60 | 7,60 | 7,60 |
The measurements carried out by the above-mentioned method with high accuracy have demonstrated the possibility of using this method for any variant of isotopic content of oxygen. Some results of the measurements are given in the (Table 1). The analysis of the Table allows us to make the following conclusions: by this method, it is possible to determine the atomic fraction of oxygen isotopes within the range of 0,0004 - 99,999%. At the same time, it is necessary to clean the mass spectrometer from background peaks and the memory effect of the previous sample. The intensity of the ionic currents must be determined with high accuracy (no less than a four-digit number). Even small inaccuracy does not give a possibility of calculation in some variants (e.g. samples № 4, 6, 9). The background superposition took place some on ionic currents entering into the formula (obviously, on the peak with the mass number m/z=32); samples № 4 and №6, whereas the memory effect of the previous sample took place on the sample № 9. The analysis of the obtained results allows (despite the abovementioned) to determine with high accuracy the atomic fraction of each isotope of oxygen even in the given case.
Chemical Sciences Journal received 912 citations as per Google Scholar report