Amin AA, Soliman MH and Shalaby AA
DOI: 10.4172/2150-3494.1000170
The efficiency of boric acid, borax and imidacloprid is evaluated for inhibition of adult house fly emergence (IC values) as dry and liquid baits. In the two cases, imidacloprid has the greatest fatal effect at both IC50 and IC90 levels followed by boric acid and then borax. In the liquid formulation, IC50 and IC90 values are (0.083 and 2.6%), (0.19 and 2.48%) and (2.6 and 16.9%) for three tested compounds respectively. The relative efficiency for imidacloprid and boric acid compared to borax (the least potent one), imidacloprid and boric acid achieved 31.3 and 13.7 times more suppression of adult emergence than borax. In the solid formulation, IC50 and IC90 values are (0.083 - 0.67%) followed by boric acid (3.7-11.6%) and borax (5.74-21.1%) respectively. It’s clear that imidacloprid 68.8 times and boric acid 1.5 times as toxic as borax. Reasons for differences in manifestation of mortality and possibilities for practical application are discussed. We conclude that the efficiency of tested compounds as liquid baits is higher than it as dry baits.
Legesse A, Adamu F, Alamirew K and Feyera T
DOI: 10.4172/2150-3494.1000171
This research was carried out to investigate key physicochemical parameters of milk samples collected from camel, cow and goat in Jigjiga district, Eastern Ethiopia. Sixty fresh milk samples were collected purposively from camels, cows and goats (twenty samples from each species) and analyzed. The results revealed that, cow milk had 6.30 ± 0.15 pH, 0.29 ± 0.04% titratable acidity, 14.6 ± 0.60% total solid, 0.75 ± 0.07% ash, 3.54 ± 0.12% protein, 5.54 ± 0.65% fat and 1.06 ± 0.03 specific gravity. Camel milk had 6.13 ± 0.11 pH, 0.36 ± 0.01% titratable acid, 13.65 ± 1.39% total solid, 0.73 ± 0.03% ash, 3.15 ± 0.15% protein, 3.93 ± 0.15% fat, and 1.03 ± 0.00 specific gravity. Results for goat milk was also recorded as 6.38 ± 0.08 pH, 0.33 ± 0.03% titratable acidity, 14.25 ± 1.16% total solid, 0.73 ± 0.07% ash, 4.62 ± 0.56% protein, 6.79 ± 0.38% fat and 1.04 ± 0.00 specific gravity. It can be concluded that goat milk had higher protein and fat content than cow and camel milk.
Yogyta Singh, Shikha Singh, Prakash Chandra and Pratima Jain
DOI: 10.4172/2150-3494.1000172
Apparent molar volume and density are reported for organic solutions of 1,4-Dioxane, Methanol, Ethanol, and Di- Methyl Sulfoxide (DMSO), and including those of the pure liquids, and their fluid mixtures at different temperatures (293.15 to 318.15 K) and atmospheric pressure. Apparent molar volumes (VÏ•) of prepared binary solutions were calculated using these density values. The value of the apparent molar volumes show expansibility at intensity for binary method, signifying that there is an extension in volume of the fusion with increasing temperature.
Areef MMH, Adel AH Abdel-Rahman M, Abdel Aleem H and Magda H Abdellattif
DOI: 10.4172/2150-3494.1000173
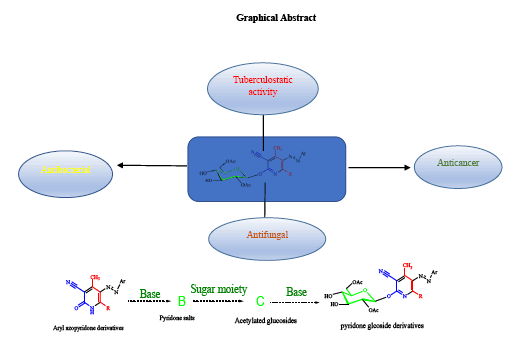
Pyridine derivatives played important roles in the last decade in to approach many and different functionalities, especially as antitumor, antibacterial, anti-fungal, and many of pharmacological activities. Novel compounds of 5-Arylazo-2- [(substituted)-3,4,5-trihydroxy-6-(hydroxymethyl) tetrahydro-2H-pyran-2-yloxy]-4,6dimethyl nicotine nitrile, (3a-e), generally called (Fluro arylazo pyridine glucosides) were synthesised via green protocol, microwave scheme 3, and the compounds were investigated on the basis of spectroscopic data (FT-IR, 1D, 13C), and antibacterial and antifungal studies had been applied.
Graphical Abstract
Rodrigo A Sarria-Villa, José A Gallo-Corredor and Martha Isabel Páez
DOI: 10.4172/2150-3494.1000174
Pine bark is a rich source of natural polyphenols, compounds which have attracted increasing attention in the fields of nutrition, health and medicine. Extractive components include large amounts of phenolic compounds. The ethanolic extract was obtained from Pinus patula bark grown in the forests of the Cauca Department in Colombia South America. The Gallic acid, catechin, epicatechin and procyanidin B2 contents in ethanolic extract were 3.12, 1.99, 0.80, and 0.71%, respectively, these compounds were determined using HPLC with UV-Vis detection. Ethanolic extract was divided using column chromatography to obtain an ethanol-soluble fraction with tannins and phlobaphenes as well as an ethanol-insoluble fraction mainly composed of Phenolic acids. Catechin and Gallic acid finally isolated were characterized using UV-Vis, IR, NMR, 13C, 1 H, HSQC, HMBC and GC-MS spectrometry.
Pruthviraj RD and Vishwa Prakash
DOI: 10.4172/2150-3494.1000175
In this study of Al 1100 alloy is selected, the corrosion test was conducted at room temp using Electrochemical studies. The corrodent medium used for the test was 1 M, 75 M, 0.5 M and 0.25 M Ammonium chloride solution. The specimen was washed with distilled water fallowed by acetone and allowed to dry thoroughly the corrodent specimen was cleaned with brush for remove adhering corrosion product washed with water and acetone, then dried. The corrosion rates are calculated using CH-Instrument. In each case the corrosion rate in ammonium chloride solution decreases and the corrosion becomes decrease in exposure time for Al alloy due to the aluminium in many induced passivation due to the formation of metal chloride layer.
DOI: 10.4172/2150-3494.1000176
[Co(L4)2Cl2] were prepared by reacting 2,3,4-trimethoxy benzaldehyde semi carbazone ligands with CoCl2.6H2O. The IR, UV, Mass and 1H NMR spectra of the complexes have been assigned. Thermo gravimetric analysis were also carried out. The data agree quite well with the proposed structures and show that the complexes were finally decomposed to the corresponding ligands. Complexes were screened for antimicrobial activities by the disc diffusion technique using DMSO as solvent. The activity data show that the semi carbazone metal complexes are more potent antimicrobials metal complexes.
Darshana Rana, Aniruddhasinh M Rana and Suban K Sahoo
DOI: 10.4172/2150-3494.1000177
A novel sensor (L) was synthesized using Schiff base reaction of pyridoxal with orthophenylene diamine and was characterized by various spectroscopic techniques such as FTIR, 1H NMR and Mass spectrometry. The cation recognition ability of the synthesized sensor was investigated by experimental (UV-VIS, IR and 1H NMR) and theoretical (B3LYP/6-31G**) methods. Among the tested anions, the developed sensor showed a naked-eye detectable color change from colorless to yellow and spectral changes in the presence of Fe(II) due to the formation of hydrogen bonded complexes with these cations followed by the partial deprotonation of sensor. With a micromolar detection limit, the developed sensor proved highly efficient and can be utilized for the colorimetric detection of Fe(II) ions.
Chemical Sciences Journal received 912 citations as per Google Scholar report