Research Article - (2022) Volume 12, Issue 1
Received: 29-Nov-2021, Manuscript No. jcde-21-48620;
Editor assigned: 30-Nov-2021, Pre QC No. P-48620;
Reviewed: 27-Dec-2021, QC No. Q-48620;
Revised: 11-Jan-2022, Manuscript No. R-48620;
Published:
21-Jan-2022
, DOI: 10.37421/2165-784X.22.12. 432
Citation: Moges, Tariku and Biruktawit G. Meskel. “Treatment of Greywater by using Banana Peel Biochar and Sand Filtration.” J Civil Environ Eng 12 (2022): 432.
Copyright: © 2022 Tariku M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Greywater treatment and reuse is becoming a significant field of research in a worldwide context of increasing water shortage. In this study the banana peel biochar was used as an alternative to treat greywater. The purpose was to evaluate and compare the performances of biochar and sand in reducing turbidity, COD, N, and P. The biochar and sand were packed to a depth of 27 cm into vertical columns with a diameter of 7 cm. The columns were fed with synthetic and real greywater. Synthetic greywater was used for optimization of biochar particle size (Fine, Medium and Coarse) and pH. Real greywater was fed to the column by taking the optimum biochar particle size and pH and its performances were compared with sand. Fine particle size (FBC) of biochar showed more removal efficiency than medium and coarse sized particles. The results of the pH optimization showed that the highest treatment was achieved at pH value of 7. FBC showed better removal efficiency than sand, with the value of 96%, 71%, 85%, and 99% for turbidity, COD, N, and P respectively. The findings of this paper indicate that transformation of banana peel into biochar have a double advantage of treating greywater and minimizing the amount of waste that is disposed into the environment.
Fine biochar • Grey water • pH • Removal efficiency • Sand
Nowadays there is an increasing interest in the reuse of wastewater in many parts of the world, including both industrial and developing countries. One of the reasons is water shortage, or too large demands of freshwater from the population. On the other side in some countries, the driving force for reuse of wastewater is environmental and economic considerations. The reuse will lower the total costs for wastewater handling, since there will be a reduced load of water to the treatment plants [1].
Greywater is a domestic wastewater, which may come from kitchen sinks, bathroom, wash basins and washing machines can collectively be called as grey water. On the contrary water from toilets containing urine and fecal matter is known as black water. Grey water constitutes about 55-75% of total household wastewater and fewer pathogens compared to domestic wastewater [2].
Degree of treatment can be decided based on the treatment quality to be achieved. Reuse of domestic waste water for potable use requires a higher degree of treatment including the tertiary treatment. But water quality for various non-potable uses like landscape irrigation, agriculture, toilet flushing and ground water recharge can be achieved more easily by using the conventional and cost effective treatment techniques like coagulation, filtration and biological treatment systems [3].
Thus, to reduce cost, treatment of greywater by natural system is gaining popularity in both developed and developing countries. These natural treatment systems such as sand/gravel filters, constructed wetlands (planted soil filters) and trickling filters are now competing with various conventional intensive technologies to treat greywater at household level and also, new technology is developed to use biochar as greywater treatment [4].
Sand filtration is the oldest wastewater treatment technology. It has been used successfully in Europe since the early 1900s and still a popular method of treating municipal water supplies and wastewater [5]. Its treating mechanism is filtration. Besides the physical filtration through the sand, an active biofilm develops. It is attached to the sand particle surface sand mineralize organic matter from the wastewater [6].
Biochar derived from biomass is defined as a carbonaceous residue from pyrolysis, including natural fires under limited oxygen. The application of biochar to the soil can improve its fertility and crop production, with the positive effect of mitigating the rising concentration of atmospheric carbon dioxide [7]. Currently, biochar is recognized as an environmental-friendly adsorbent to abate organic pollutants [8]. Due to having a large surface area (100–1000 m2/g), low density and high porosity [9] which makes it an efficient adsorbent and good biofilm carrier. The unique properties of biochar enhance water and wastewater quality in onsite systems.
Banana is a major fruit crop grown in many developed and developing countries in terms of consumption and production, among the horticultural crops. The average mass of peel of banana is about 25 to 20% of total mass of banana, a 145million tonnes of Banana is consumed annually worldwide, the amount of banana peel will be approximately 40million tonnes [10]. This large amount of banana peel can be recovered for further use as biochar rather than disposing it into landfill. Furthermore, it is shown that N and P adsorbed from wastewater are slowly available to plant and also used for soil amendments. Thus, this study aims to investigate the treatment efficiency of banana peel biochar for grey in comparison with the well-known sand filtration.
Biochar preparation
The biochar used in this study was produced from banana peel and prepared in the laboratory using pyrolysis process. The peel was collected from local juice bar and dried in the sun for days. After the banana peel was dried, it was crushed with a blender to prepare three different sizes of biochar. The first particle size was coarse biochar (CBC) which is > 3 mm, second biochar size was medium biochar (MBC) with size of 1-3 mm, and the last particle size was < 1 mm, which is fine biochar (FBC). Then the three different particle sizes were carbonized by an electric muffle furnace, at temperature of 450°C for 1.5 hour. Then the carbonized biochar cooled at room temperature and washed with distilled water to remove the very fine particle and oven dried at temperature of 100°C for 3 hours (Figure 1).
Biochar characterization
Following, Novak JM, et al. [11], biochar pH and Electrical Conductivity (EC) were measured in 1:10 biochar to distilled water mass ratio after shaking for 30 min. After this, samples were allowed to stand for 30 min and then pH and EC was measured. The pH reading was taken using HI 8010 model and EC were also measured using JENWAY 4330 conductivity meter.
Elemental analysis was also performed using a CHNS elemental analyzer (FlashEA 1112) to analyze the elemental content of carbon, hydrogen, nitrogen, and Sulphur present in the banana peels; the oxygen content was obtained by mass difference (O =(100 - C - H - N -S) [12].
The morphological properties of biochar were analyzed by Scanning Electron Microscopic (SEM) imaging. A range of SEM images (Magnification: 500X to 2000X were captured with a JEOL JSM- 6490 operating at 20 KV at Ethiopian Leather Industry Development Institute, Addis Ababa. Image analysis was done with Image J version 2.0 with appropriate threshold and size range values.
The specific surface area of biochar was done using air permeability apparatus and also bulk density was determined by dividing the dry weight of the filter medium by the volume occupied by the medium.
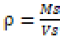 (1)
(1)
ρ= g cm-3 bulk density
Ms= g, mass of solids
Vs= cm3, volume of solids
Sand preparation and characteristics
Sand was purchased from a local building material supplier and washed by distilled water to remove very fine particles and dissolved organic matter. It was also left to air to remove excess water oven dried at 105°C.
The particle-size distribution of the sands on a weight basis was analyzed in triplicate by conventional dry-sieving techniques. The grain-size distribution plots were used to estimate d10 (10% of the sand by weight is smaller than d10) and d60 (60% of the sand by weight is smaller than d60). The uniformity of the particle-size distribution (the uniformity coefficient) was calculated as the ratio between d60 and d10.
To determine effective size and uniform coefficient of particle, three homogeneous and representative 1000 g samples were taken at each sampling event, dried and sieved through seven stainless steel screens ranging from 0.075 to 4.75 mm pores. Then, each screen was weighed to determine the retained sand and the particle size distribution curve, which allowed the computation of UC.
Furthermore, the sand SEM, bulk density and specific surface area were analyzed following the method explained under biochar characterization.
Experimental setup
The experimental setup consisted of six plastic cylinders with diameter of 7 cm and a total height of 36 cm each. The bottom of each column had a small 0.5 cm diameter outlet. Each filter cylinder was filled with three layers (Figure 2a). These were top gravel middle biochar or sand and bottom gravel. The depth was fixed by taking the proportional suggested by Berger C [13]. Based on that, the gravel filled with 1.4 cm at the bottom, then 27 cm of depth were filled by biochar or sand and added to the column spoon by spoon in order to pack them as densely as possible, but in case of FBCS the 13.5 cm depth was filled by FBC and the rest depth was filled by sand media. For the same reason, the outside of the column was knocked after each spoon. A layer of 1.4 cm top gravel was added and finally the whole column was wrapped to aluminum foil in order to prevent light penetration to avoid the growth of algae (Figure 2b).
Synthetic greywater preparation and composition
The synthetic greywater (SGW) used in order to control the quality of the test of greywater, to ensure repeatability of the quality, and to optimize different parameters. The ingredients used for SGW were 0.4 g/l sunflower oil (Tena, Ethiopia), 0.16 g/l shampoo (Organza, rose flowers, U.S.A), 0.16 g/l dishwashing gel (SHAGAN, U.S.A), 0.16 g/l washing powder (Ariel, Germany) and 2.5 g nutrient broth. The nutrient broth was prepared in a laboratory using 10 g/l of beef extract, 10 g/l of peptone and 5 g/l of sodium chloride [14]. Nitrogen and Sulphur were not detected in the peel. EC and pH were found to be 3.36 μS/cm and 9.93 respectively. Then, it was completely mixed by flocculator with distilled water. The prepared 1 L of SGW was used only for one day of sample test.
Real greywater: Greywater with a volume of 5 L was collected at working days from Kebele house that was near to AAiT. The samples were collected directly from the shower or bathtub, laundry kitchen water was collected from the kitchen sink and it were analyzed on the day of production within a maximum of 24 hours duration.
Analytical method
Physiochemical analyses of RGW and SGW were conducted for the selected parameters: pH, turbidity, COD, N, and P. Samples of inflow greywater were taken at the time of feeding and outflow samples were taken immediately after enough water was accumulated in the outlet recipient. The analysis was done for four days and run in triplicate then the average values were taken. The pH, conductivity and turbidity were determined in situ using a calibrated Hanna educational pH meter- HI 8010, JENWAY 4330 conductivity meter and Hanna HI 93102 turbidity meter respectively.
According to Ahmadvand report the contents of N, H and O decreases as pyrolysis temperature increased from 400 to 700°C, whereas carbon increased with higher pyrolysis temperatures. At this stage, some of the nitrogen oxidized into nitrogen oxides and Sulfur volatilized and his finding concluded that carbonization was promoted with increasing pyrolysis temperature. Hydrogen and oxygen losses at high pyrolysis temperature were due to the cleavage and breakage of weak bonds within the biochar structure.
Physical properties of FBC compared with sand
The physical characteristics of biochar were 400 kg/m3 and 118 m2/g for bulk density and specific surface area respectively. It revealed that biochar was lighter and had larger specific surface area compared to sand (1600 kg/ m3 bulk density and 0.156 m2/g specific surface areas). And both N and P was analyzed by Palintest photometer 7100 (UK).
Biochar characteristics
The elemental analysis of banana peel biochar as shown in Table 1 had high carbon percentage whereas hydrogen was detected in low concentration.
| Elemental Composition of Banana Peel Biochar % | ||||
|---|---|---|---|---|
| N | C | H | S | |
| - | 69.99 | 2.33 | - | |
The other physical characteristic of biochar and sand was pore structure. As shown in Figure 3a SEM image of biochar had random pore structure on the surface and pore appeared over the surface but sand particles (Figure 3b) had solid structure with limited occurrence of micro pores. This means that a filter made of biochar would have better capacity to hold water in macro pores than a sand filter and has also better capacity to form biofilm in the pores without clogging. Therefore, biochar has more suitable surface conditions for bacterial attachment and biofilm development than sand filter, which leads to an efficient biological degradation of organic matter and nitrification.
Sand characterization
The sand selected for the experiment had effective grain size (d10) and (d60) 0.35 mm and 0.9mm respectively as shown in Figure 4. According to Danish EPA guidelines (EPA 1999), d10 and d60 should be in the range of 0.3-2.0 mm and 0.5-8 mm respectively. Uniformity coefficient (d60/d10) should be less than four, in order to secure an adequate hydraulic conductivity.
Performance of FBC, MBC and CBC using SGW
The COD reduction efficiency of FBC, MBC, and CBC at the last day of the experiment was 84.1%, 53.8% and 48.3% respectively. The turbidity reduction potential of FBC, MBC, and CBC were found to be 91%, 57% and 18% respectively. It were also found that FBC, MBC, and CBC were able to remove 91.7 %, 38.5% and 19% of N; and 75%, 32% and 18% of P respectively, as shown in Figure 5.
CBC and MBC particle sizes had lower efficiencies when compared with FBC, due to large macropores in the filter. Under this condition, greywater passes through the filter media quickly (0.20 cm3/s and 0.17 cm3/s for CBC and MBC) without enough contact time. However, in FBC filter media, the average discharge was 0.09 cm3/s which had good contact time and good treatment efficiency. According to Mohanty and Boehm and Kolodynska, smaller size particle is efficient in removing pollutants from wastewater, due to high micro pore and optimum surface area which is used to retain water for longer time.
Effect of pH on removal process using SGW
As shown on Figure 6a, N removal increased within pH 5 - 7 (from 88 to 98.4%) and declined after pH 8(from 77.6% to 50.2 %). High N removal was achieved when pH was 7. Phosphorus removal by biochar was observed at pH values ranging between 5 and 10. Figure 6b shows that as the solution pH increases in the range of 5-7, the removal increased gradually and attained its maximum value (82.7%) when the pH value was 7. However, between pH value of 8-10 decrease in the removal of phosphorous was observed with final removal of 23.6%.
Figure 6c depicts the effect of pH on COD removal. The COD removal capacity of FBC was high within pH value 5 - 7 then decreased as the pH increased from 8 - 10. At pH 7 high percentage of COD removal with value of 84.2% was achieved.
Figure 6d shows the graphical representation of turbidity removal percentage. It was observed that the removal of turbidity increased with increase in solution pH. After the pH of 7 the removal of turbidity was decreased with the increase in solution pH. The maximum removal efficiency of turbidity at pH 7 was 90.7%.
Figure 6. Effect of pH on a) N removal b) P removal c) COD removal and d) turbidity removal.
Comparison of FBC and sand using RGW
Batch experiments were conducted to see the efficiency of FBC and Sand to remove turbidity, COD, N and P from the RGW. Accordingly, turbidity removal of 96% by FBC and 90% by sand were achieved at the end of the experiment. Meanwhile, COD, N, and N removal of 71%, 85%, and 99% respectively using FBC and 58%, 66%, and 32% respectively using Sand were achieved (Figure 7).
Various researchers investigated the effectiveness of sand filters for treatment of wastewater at laboratory scale, and suggest that sand filters are capable of removing turbidity up to 88%. Farooq S and Al-yousef AK [15] conducted a pilot study using slow sand filtration with effective sand sizes of 0.31 and 0.56 mm for the treatment of secondary chlorinated effluents, and achieved 50-67% COD removal. The finding of this paper is not far from the above literature. The turbidity removal was 90% and COD removal was 58%. The COD reduction by biochar filter made from coconut shells was 73% and by sand 58% as reported by Demirbas A [16].
FBC had high efficiency than sand due to different characteristics such as porosity, specific surface area and reactivity, adsorption capacity and ability to promote biofilm development for biological breakdown of organics. Biochar filters are characterized by large specific surface area and high porosity, which provides better absorption capacity and thus achieves a greater reduction of pollutants from start-up compared with sand. FBC had a specific surface area of 118 m2/g and sand had small surface area of 0.156 m2/g. This indicates that the flow velocity through FBC is slower than sand, and it had higher residence time. This resulted high efficiency for FBC filter.
In addition, filtration mechanism of biochar depends on the characteristics of large specific surface areas and rich pore structures enhance the physical adsorption capacity of biochar, and the rich pore structures help to adsorb the organic matter with the same molecular weight. Also, electrostatic attraction ability on the surface of biochar plays a very important role in the adsorption of pollutants [16]. The surface electricity of biochar is negative, so it has a good adsorption performance for positive ions [17]. But in case of sand media filter physical filtration is the main removal mechanism of impurities by mechanical straining. Moreover, organic impurities could be reduced chemically by oxidation and biologically by aerobic degradation [18].
In this study SGW and RGW were used and the biochar was produced from banana peel with three different particle sizes. FBC showed good removal efficiencies with average performances of 88% for turbidity 54.8% for COD, 62.2% for N and 52% for P respectively. The effect of pH on FBC was investigated by changing pH value of SGW and the result showed that the highest removal of turbidity (89%), COD (55%), N (94%) and P (78.8%) were achieved at pH value of 7. Comparison of FBC and sand performances using RGW for different parameters revealed better pollutant removal efficiency of FBC: 95% for turbidity, 99% for N, 85% for P and 71% for COD.
The removal efficiency of FBC filter is high due to its high adsorption capacity. It is also potentially good in removing organic and inorganic substances from greywater and contributing to greywater recycling and safer environment.
The authors are thankful to School of Civil and Environmental Engineering; Addis Ababa Institute of Technology (AAiT), Addis Ababa University for providing necessary facilities and supports to complete this study.
Google Scholar Crossref PubMed
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref Indexed at
Google Scholar Crossref PubMed
Journal of Civil and Environmental Engineering received 1798 citations as per Google Scholar report