Research Article - (2021) Volume 11, Issue 2
Received: 22-Jan-2021
Published:
09-Feb-2021
, DOI: 10.37421/2165-784X.2021.11.375
Citation: D’Alonzo, Giovanna. “Change in the State of Matter
of Metals and Solids Using a Reticular Atomic Filter in Vacuum and Creation of
New Materials: A Hypothesis of Change B-C and Si-BP.” Civil Environ Eng 11
(2021): 375.
Copyright: © 2021 D’Alonzo G. This is an open-access article distributed
under the terms of the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.
In a small temperature range, at temperatures below the temperature sufficient for the passability of the electronic cloud of atoms that make up a solid in vacuum experiments, a “fusion” could occur between the atom that characterizes the solid and the atom or ion that composes the gas. The procedure must be performed in a vacuum with the help of Special Forces, including the pressure force of a piston, the increase in the power of the natural kinetic energy of the gas using suitable instrumentation, harmonic oscillations of the filter, and other forces to be applied about the specific experiment to be carried out. The merger between the atom constituting the filter, and the atom formatting the gas could lead the system with its complex mechanism of energies and forces to opt for the minimum energy configuration. It i.e., could happen that the two nuclei of the two atoms merge. In this way, a change in the state of matter could occur; even the crystalline form of the solid could change. If the gas consisted of hydrogen atoms, the atom constituting the solid after this transformation could increase its atomic number by changing its state of matter even in its crystalline lattice shape. Some application hypotheses are the transformation of Boron into one of the various forms of Carbon and the change of Silicon into black phosphorus.
State of matter change • Solids • Metals • New materials.
The hypothesis of the adaptability of the electronic cloud of the atoms constituting a solid in the vacuum under certain temperature conditions and additional forces using special tools in the void is based on the search for the temperature sufficient for the adaptability of the electronic cloud of the atoms constituting the solid [1]. At temperatures slightly below the adequate temperature, a “fusion” temperature is assumed between the atoms making up the solid and the atoms/ions making up the gas. In this narrow temperature range, between the “fusion” temperature and the sufficient temperature, we can assume that the atom/ion making up the gas remains trapped inside the atom making up the solid and that in a short time, the system tries to balance itself around the states with minimal energy and, therefore, we can hypothesize a “fusion” between the nuclei and a change of state of the metal. The total power of the gas is the ionization energy if the gas is formed of ions, from which the gravitational energy and the increase in the strength of the natural kinetic energy of the gas due to special instruments used in vacuum are subtracted, to which is added the difference between the internal energies respectively of the atom constituting the solid and of the atom or ion forming the gas [2-6]. The difference between the internal energy of the atom making up the solid and the atom or ion making up the gas is negligible. The total power is equal to the average gas energy. This equation concerning temperature is integrated. The integration extremes are the “fusion” temperature between the atom constituting the solid and the ion or atom formating the gas and the temperature sufficient for the validity of the electronic cloud of particles constituting the solid. The “fusion” temperature is obtained from this integration. Some application hypotheses are the transformation of Boron into one of the various forms of Carbon and the change of Silicon into black phosphorus.
The “fusion” temperature between the atom making up the solid and the gas
Total energy is the following [7]:
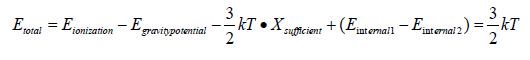
Internal energies are negligible [8]. We integrate the equation as a function of temperature with integration extremes the “fusion” temperature and the sufficient temperature:
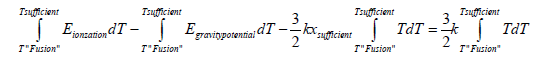
Therefore, the “fusion” temperature is:
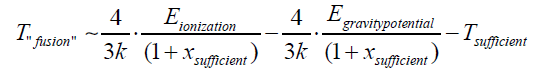
So,
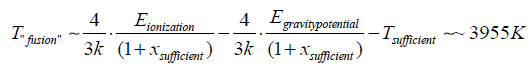
In the temperature range 395.5 K <T <396.28 K, a “fusion” could occur between the atoms making up the solid and the hydrogen ions making up the gas.
First hypothesis: Boron and Carbon
An experiment that I would like to carry out concerns the use of a metal boron filter [9]. Boron is a very hard and resistant solid of black color, has various crystalline forms, and is also found in the amorphous state, and is similar to silicon [10]. When boron is very pure, it is stable at room temperature, but as the temperature rises, it reacts with oxygen. So the experiment must be done in the absence of air in a vacuum as I elaborated in my previous article [11]: “The necessary equipment would generally be the following: in a vacuum, a steel or glass container for molecular oxygen, H + ions and electrons; the upper surface of the container should be a led piston which in addition to overcoming the Coulomb forces in the crystal lattice would have two other tasks, induce water oxidation and prevent the recombination of ions and hydrogen electrons before filtration; special tools such as fans should be incorporated into the piston; they should be able to produce an energy that could contribute, through the distribution of molecules and through mathematical passages, to an adequate temperature; the steel or glass container must have a removable bottom; the solid should oscillate harmoniously by some micrometers to facilitate filtration; an instrument capable of heating the solid at a temperature of 396.28 K; two thin metal grids to support the solid; the solid should be placed on an instrument capable of sucking H + ions and electrons which will be "filtered" by the solid and collected in a steel container; the suction tool should take more time in the direct direction and a shorter time to aspirate in the opposite direction to avoid the obstruction of the solid mainly in the empty spaces of the crystal lattice and also the piston should move some micrometers from the bottom to the top in the very moment in which the aspiration takes place in the opposite direction with the same instrument to avoid the obstruction of the empty spaces and the breaking of the solid” [1]. The atoms that make up a metal boron filter could fuse with a hydrogen ion and absorb an electron at a temperature higher than the “fusion” temperature and lower than the temperature sufficient for the electron cloud to pass through [12]. At this point the atoms that make up the solid will try in the shortest possible time to reach a state of minimum energy and, therefore, a transformation of the shape of matter of boron could take place: the nuclei could absorb the ion, and so the atomic number increases by one [13]. What could be achieved could be the various forms of carbon, most likely graphene, or a new material as described in the following Table 1 [14,15].
| Atoms | Atomic Number | Allotropes | Melting Point | State of Matter Change |
Atomic number | Allotropes | Melting Point |
|---|---|---|---|---|---|---|---|
| B | 5 | Amorphous Boron | 2349 K | C | 6 | Graphite | 3773 K |
| Metallic Boron | Diamond | ||||||
| Amorphous | |||||||
| carbon | |||||||
| Fullerenes | |||||||
| Graphene | |||||||
| New Materials |
Of course, likely new materials can also be obtained by iterating this process and, therefore, using, for example, a graphene filter. The latter would absorb a hydrogen ion and an electron at a temperature higher than the "fusion" temperature and lower than the temperature sufficient for the electron cloud to pass through. Its atoms would increase in atomic number by one according to the same transformation. This transformation of the state of matter could be iterated for many atoms on the periodic table of elements [16]. The "fusion" temperature depends only on the ionization energy, the gravitational potential energy, the temperature sufficient to allow the electronic cloud to be crossed, and the numerical variable xsuff ; as the difference between the internal power of the atom constituting the solid and the atom or ion formating the gas is negligible:
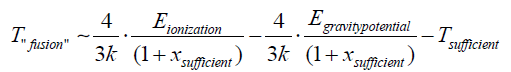
( Eintenal1 - Einternal2 ) negligible energy
The data of the “fusion” temperature and of the temperature sufficient and of the numerical variable xsuf relative to the hydrogen ion, that is: Tfus~ 395.5 K;
Tsuf~ 396.28 K; xsuff ~ 252 are suitable for any type of solid used as a filter [1].
Second hypothesis: Silicon and black phosphorus
Another experiment that I would like to carry out is to use a pure silicon filter to observe if a transformation of the state of matter into black phosphorus is possible, as black phosphorus is a rare and very important metal, or to note if new materials are formed, as described in the following Table 2 [16]: Instead, using a black phosphorus filter with its solvation shell, it could be observed whether after the capture of hydrogen ions and electrons, a transformation of the state of matter of black phosphorus occurs, associated with a change of the solvation shell of solvation becomes suitable for the new solid that would thus be created [17]. This last solid, thus created could be used as a filter to discover still other new materials. The experiments to be carried out with this method are manifold, and one can start from many solids [1].
| Atoms | Atomic Number | Melting Point | State of Matter Change | Atomic number | Melting Point |
|---|---|---|---|---|---|
| Si | 14 | 1687K | BP | 15 | 873K<T<1273K |
| New Materials | (Black Phosphorus) |
Other transformation hypotheses
Obviously you can iterate this procedure to many elements of the periodic table starting from metals or suitable solids whose main characteristics must be hardness and resistance.
This study is based on research of a "fusion” temperature between the atom constituting a solid and the atoms or ions that make up the gas in vacuum experiments using applied forces and added energies. At temperatures between the "fusion" temperature and the temperature sufficient for the electron cloud to be crossed, this "fusion" can occur, and the atoms that make up the solid could search for the minimum energy state in a short time and a change in the shape of matter of the solid itself [18,19].
The search for new materials has become widespread in recent years and is of fundamental importance for the metallurgical industry. This hypothesis of the transformation of the state of matter of solids can lead to discovering new materials. This hypothesis can also lead to the realization of already existing materials in an alternative way. Therefore, rare and useful materials for the metallurgical industry could increase its availability. For example, black phosphorus is very important and rare material. With this hypothesis, it could become easier to find and more abundant. Furthermore, this hypothesis, when for example, black phosphorus with its solvation shell is used, could transform not only the black phosphorus solid but also its solvation shell in such a way as to make it suitable for many other metals. Starting from a appropriate metal, this transformation could be iterated for many elements.
Journal of Civil and Environmental Engineering received 1798 citations as per Google Scholar report