Research - (2020) Volume 10, Issue 4
Received: 15-Jun-2020
Published:
07-Jul-2020
, DOI: 10.37421/2165-8064.2020.10.409
Citation: Onuegbu Genevieve, Nnorom Onyekachi, Okonkwo Samuel Nonso and Ojiaku Pascal. Acid-Base Indicator Properties of Dye from Local Plant: The Rosella Calyces (Hibiscus sabdariffa). J Textile Sci Eng 10 (2020) doi: 10.37421/jtese.2020.10.409
Copyright: © 2020 Onuegbu G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.
The dye from the Calyces Hibiscus sabdariffa (Zobo) was extracted and used as acid-base indicator for the standardization of acid and determination of the molar masses of some selected acids. The Calyces Hibiscus sabdariffa was peeled, washed and heated in an oven at 60°C. It was ground into powder and soaked in hot and cold ethanol for the extraction. Part of the extract was filtered and concentrated by subjecting the extract to slow heating which yielded 1.5 kg of the Hibiscus sabdariffa indicator. Hot and cold extracts of the Hibiscus sabdariffa were used for the standardization of acid with 0.03 M concentration of the bases. On the preliminary test carried out on basic medium, the calyces Hibiscus sabdariffa indicator turned red in acidic condition and green in basic medium, both hot and cold indicators yielded sharp and intense colours at the end points during titration processes. However, there were no colour changes for weak acid versus weak bases (H2C2O4/Na2CO3 and H2C2O4/B4Na2O7). The mean volumes of the acids used were determined and used for the determination of the molar concentration and mass concentration of all the acids used in the research work.
Indicator • Acids • Bases • Hibiscus sabdariffa • Rosella Calyces • Dye
Extensive studies have been made on the use of natural products which are readily available, easily prepared, eco-friendly, less hazardous and of low cost, as indicators in acid-base titrations. These products can serve as substitutes for the synthetic compounds which are costly and harmful. These natural products can be obtained from various parts of plants like leaves, roots, fruits and stems. Example of such plant is Hibiscus sabdariffa known as “Roselle” (Zobo), which is rich in vitamins, natural carbohydrate, protein, tannins, gums and other antioxidants including minerals [1,2]. Hibiscus Sabdariffa plant has so many applications industrially and medically. Industrially, it can be used as spices for soup, sauces, wine and juice [3], infusion (herbal tea) [4], and dyes [5]. Medically, it can be used for curing allergies, high blood pressure [6], diabetes mellitus [7], for the stimulation of intestinal peristalsis [6] and for the reduction of serum cholesterol in humans [8].
In rural areas, the calyces Hibiscus sabdariffa can be used to improve the economic value by using it as acid-base indicator in Chemistry Laboratory in Secondary Schools. This is as a result of the presence of anthocyanin compounds in rosella flowers.
Several studies have been reported by many investigators on the effectiveness of the indicator prepared from the calyces Hibiscus sabdariffa for acid-base titration. The indicator changes colour over a range of hydrogen ion concentration which is expressed as the pH ranges of blue, red and colourless [9]. A study has been carried out to compare the properties of acid-base indicator of Rose, Allamanda and Hibiscus flowers [10]. Similarly, the acid-base indicator properties of dyes from Basella alba and Hibiscus sabdariffa has been determined [11]. Rosella flower has been used as alternative indicators of Blue and Red litmus [12,13]. Natural acid indicator has been isolated from the flower sap of Rosella [14,15].
Anthocyanin has been extracted from the corolla of Roselle and used as acid-base indicator. In the study, the properties of Hibiscus rosella indicator with phenolphthalein and methyl orange indicators were compared. The performance of the natural indicator was found to be similar to that of methyl orange as the results showed that the Roselle’s corolla indicator gave red colour in acidic solution and green in basic solution [16]. In another study, it was found that a solution of Hibiscus sabdariffa crude anthocyanidins can be employed as the end-point indicator in complexometric and weak acid-weak base titrations because the end results were identical to those reproduced with standard end-point indicators, erichrome black T and phenolphthalein [17].
The objective of this research was to extract the dye (acid-base indicator) from calyces Hibiscus sabdariffa and to determine the acid-base indicator properties of the dye extracted from the calyces Hibiscus sabdariffa, for the determination of the molar concentration (standardization) of the acid and the mass concentration of the acid.
Collection of plant Materials
The calyces Hibiscus sabdariffa was purchased at Ekeonunwa Market, Owerri, Imo State, Nigeria, in the month of October, 2019.
Reagents and Apparatus
The reagents used were Oxalic acid (H2C2O4), Sodium hydroxide (NaOH), Sodium carbonate (Na2CO3), Disodium borate (B4Na2O7), Hydrochloric acid (HCl) and they were obtained from Mr Nti’s laboratory, a Technologist in School of Agriculture and Agricultural Technology, Federal University of Technology Owerri, Nigeria. The reagents were of analytical standard.
The apparatus used were burette, conical flask, volumetric flask, beaker, funnel, automatic pipette, dropper, distillation apparatus, auto digital pH meter, heating mantle, oven, laboratory meter, electronic weighing balance, retort stand with clamp, wash bottle, spatula, stirrer, soxhlet extractor, desiccators, mechanical blender.
Preparation and Extraction of the Indicator from the calyces Hibiscus sabdariffa
The flowers were washed with distilled water, dried in hot air oven at 40°C and ground into powder with mechanical blender. About 1.5 kg of the calyces Hibiscus sabdariffa was weighed and treated with two litres of ethanol at 70°C in a Soxhlet extractor for 72 hrs. The extract was filtered and concentrated in vacuum and finally dried in a desiccator to remove residual water which yielded 200 g of the crude extract. For the hot extract, 40 g of the powder was weighed using an electronic weighing machine. This was dissolved in 80 ml of ethanol which boiled for 30 mins. It was extracted and filtered to obtain the dye solution which was concentrated to produce acid-base indicator.
For the cold extraction, the ground calyces Hibiscus sabdariffa was dissolved in 60 ml of cold ethanol and left overnight, the solution was later extracted, filtered and concentrated by heating to produce an indicator for acid-base titration (Table 1 & 2).
| Titration | Acid /Base | Rapid change of pH |
|---|---|---|
| Strong acid/Strong base | HCl/NaOH | 3.4 to 6.4 |
| Strong acid/Weak base | HCl/Na2CO3 | 3.4 to 5.2 |
| Strong acid/Weak base | HCl/B4Na2O7 | 3.4 to 4.5 |
| Weak acid/Strong base | H2C2O4/NaOH | 3.4 to 9.0 |
| Weak acid/Weak base | H2C2O4/Na2CO3 | 3.4 to 3.82 |
| Weak acid/Weak base | H2C2O4/B4Na2O7 | 3.4 to 3.7 |
| Dye stuff | Colour of the dye | Basic | Acids |
|---|---|---|---|
| Hibiscus sabdariffa | Wine | Green | Red |
The calyces Hibiscus sabdariffa extract was respectively added to 0.03 M of the bases which brought about the changes in colour of the mixture (end point). At these end points, the volumes of the acids used were recorded.
Standardization of the Acid used
The mean volumes of the acids were used to determine the molar concentration and mass concentration of all the acids used in this research work. Equation 1 was used in the determination of the molar concentration
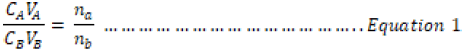
where;
CA = Concentration of Acid
CB = Concentration of Base
VA = Volume of Acids,
VB = Volume of Base
na = number of moles of acid
nb = number of moles of bases.
Mass Concentration = Molar Concentration × Molar mass
The use of Hibiscus sabdariffa as indicator in acid-base titration cannot be over emphasized in acid-base titration. The ethanol extract showed wine colour in acid solution, green in basic solution and it was employed for the standardization of all the acids used and for the determination of the mass concentration.
Journal of Textile Science & Engineering received 1008 citations as per Google Scholar report