Fatiha EL Babili, Amandine Guillouty, Halova-Lajoie Barbora, Caroline Vincent and Nathalie Sejalon-Delmas
DOI: 10.4172/2161-0444.1000468
The leaves of Hydnophytum formicarum Jack belonging to the family Rubiaceae, a tropical tree commonly found in eastern and southern Thailand, South Asia, Papua New Guinea and the Pacific Islands, were studied for these antioxidant compounds. Sterols, flavonoids and phenolic compounds appear to be good markers for this species. Chromatographic and histochemical techniques were used to analyze its secondary metabolites and to localize these molecules in the leaves of H. formicarum and its petiole. With histochemistry, we can locate the phenols in situ by an iron chloride reagent, which gives a black color, especially in fibers, hypodermis and xylem. Flavonoids are detected histochemically using a potassium hydroxide reagent to obtain a yellow coloration under visible light. Histochemical studies of H. formicarum showed a high concentration of natural antioxidants in the central cylinder, particularly in the vascular bundle, whereas condensed catechic tannins were mainly detected in collenchyma, fibers and also in xylem. The high levels of phenolic compounds and their localization in the conductive tissues allow us to explain the traditional medicinal use carried out in Thailand with this tree because, very often, compounds with antioxidant properties prove to be good anti-cancer agents.
Meihao Liang, Wenhai Huang, Beibei Wang, Wenhua Wei, Chixiao Zhang, Zhimin Zhang and Zhengrong Shen
DOI: 10.4172/2161-0444.1000469
Alzheimer's disease (AD) is a progressive neurodegenerative disease leading to the irreversible loss of brain neurons and cognitive abilities. Multiple factors, such as acetylcholinesterase (AChE), metal ions and amyloid-β (Aβ) have been considered play an important role in the pathogenesis of AD. In this work, AChE and metal ions, both of which are also associated with the deposition of Aβ in the brain, were selected as targets simultaneously. 22 compounds were rationally designed by hybridizing AChE inhibitor rivastigmine and metal chelator 2-hydroxyacetophenone, in a hoping that these compounds could be as a substrate and inhibitor of AChE, while the subsequent enzymatic hydrolysis products by AChE could be as a metal ion chelator. Thus these 22 compounds were synthesized and their biological activities against AD were evaluated in vitro. The results showed that compound w8 presented the best inhibitory activity of AChE (IC50=31.9 μM), and the representing enzymatic hydrolysis products 7f exhibted the metal chelating function. Furthermore, both 7f and one of the targeted compound w15 could inhibit the aggregation of Aβ.
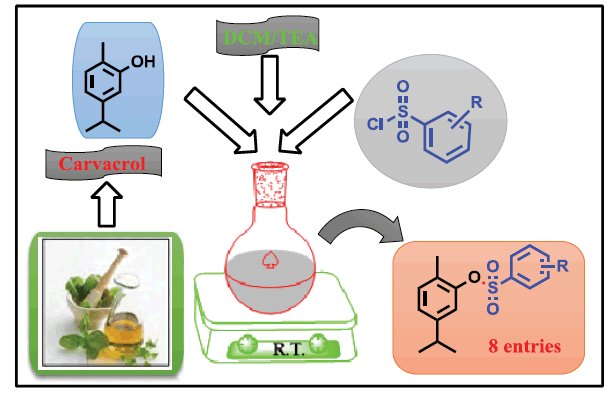
Suresh D Bagul, Jamatsing D Rajput, Manohar M Patil and Ratnamala S Bendre
DOI: 10.4172/2161-0444.1000470
In the present investigation, we report eight novel benzenesulfonate derivatives of carvacrol prepared by using sulfonyl chlorides and carvacrol. Their structures were investigated on the basis of modern sophisticated analytical techniques such as 1H and 13C NMR, LC-MS and FT-IR spectroscopy. The synthesized derivatives are screened for their antioxidant test by DPPH radical scavenger assay. Among the tested compounds, 6g and 6h have emerged as better antioxidants.
DOI: 10.4172/2161-0444.1000471
Ipomea ochraceae is from the family Convolvuliacea. It is mainly referred to as weed. The whole plant was air-dried, crushed into powdery form and then extracted using ethanol. The purpose of this study is to determine its antimicrobial activity and fatty acids from the constituents derived from GC-MS analysis.
Nagina Naveed Riaz, Fazal-ur-Rehman M and Muhammad Mahboob Ahmad
DOI: 10.4172/2161-0444.1000472
β-amino acids are an important class of macromolecules. They play role in life for survival. β-amino acids gained significant interest due to their interesting pharmaceutical uses as hypoglycemic, antiketogenic characteristics, sterile and antifungal activities, anthelminthic as well as potent insecticidal characteristics. These are vital building blocks for the preparation of pharmaceutical and agrochemical target molecules. These are utilized in development of drugs, bimolecular structure and molecular recognition. They are also important in treatment of different diseases which are viral to human health. They play important role in regulation of nutritional metabolism and immunity. It has also led to their wider adoption as intermediates in new drugs and has been a focus of considerable attention in medicinal chemistry. β-amino acids have found extensive applications as components of biologically active peptides and small molecule pharmaceuticals. Synthetic derivatives of biologically relevant peptides incorporating β-amino acids often display interesting pharmacological activity, with increased potency and enzymatic stability. β-peptides participate in arrangement of incredible stable auxiliary structures. This review summarizes recent developments about the different roles of β-amino acids in human biology and some implications in medicinal chemistry.
Mehran Miroliaei, Peymaneh Shafaei, Akram Aminjafari, Danial Barati, Riley Meekins, Safaa Kader, Kostelnik J. Colton and Esmaiel Jabbari
DOI: 10.4172/2161-0444.1000474
The present communication reports on changes in the secondary and tertiary structures of Hemoglobin (Hb) upon fructation. The presence of Lemon balm (LB) provided significant protection toward the formation of early (HbA1c) and advanced glycation end products (Hb-AGEs), as evident from circular dichroism (CD) and fluorescence studies. The degree of AGE modification and its inhibition were characterized with intrinsic fluorescence, the fibrillar state, and the exposure of hydrophobic clusters. An increased signal of intrinsic fluorescence was observed upon glycation, particularly in the later stage of fructation. Glycation mediated fibril formation and its inhibition were assessed by Thioflavin T (ThT) assay. These sequence of events were parallel with an increase in β-sheet content from ~2 to 13% for Hb-AGE, as evidenced by Congo red binding and CD. Furthermore, the late stage of glycation was also marked by an increase loss of heme moiety, confirming the relatively high affinity of ANS toward glycated-globin which was mitigated by applying the balm extract. However, the activity of balm can be attributed to inhibition of oxidative stress and free radicals derived from Hb-AGE, or chelation of metal ions producing in Fenton reaction. Overall, the presence of LB provided significant protection against AGE-induced deleterious processes, can qualify the herb as an effective AGE-inhibitor with potential prevention toward diabetic complications arising from Hb glycation.
Medicinal Chemistry received 6627 citations as per Google Scholar report