Haicheng Liu, Yushi Futamura, Honghai Wu, Aki Ishiyama, Taotao Zhang, Tao Shi, Qunxiong Zheng, Masato Iwatsuki, Satoshi Satoshi Ã…ÂŒmura, Hongbin Zou and Hiroyuki Osada
Background: Malaria is one of the most devastating parasitic diseases, yet the discovery of antimalarial agents remains profoundly challenging. Very few new antimalarials have been developed in the past 50 years, while the emergence of drug-resistance continues to appear.
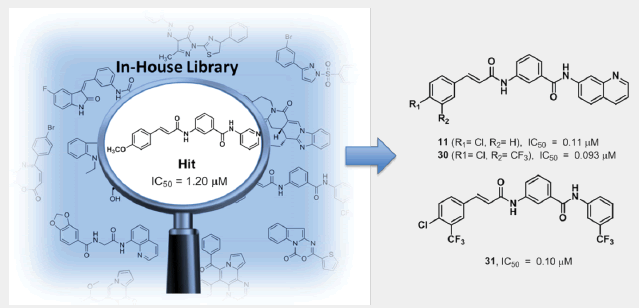
Objective: This study focuses on the discovery, design, synthesis, and antimalarial evaluation of 3-cinnamamido-N-substituted benzamides.
Method: In this study, a screening of our compound library was carried out against the multidrug-sensitive Plasmodium falciparum 3D7 strain. Derivatives of the hit were designed, synthesized and tested against P. falciparum 3D7 and the in vivo antimalarial activity of the most active compounds was evaluated using the method of Peters’ 4-day suppressive test.
Results: The retrieved hit compound 1 containing a 3-cinnamamido-N-substituted benzamide skeleton showed moderate antimalarial activity (IC50=1.20 μM) for the first time. A series of derivatives were then synthesized through a simple four-step workflow, and half of them exhibited slightly better antimalarial effect than the precursor 1 during the subsequent in vitro assays. Additionally, compounds 11, 23, 30 and 31 displayed potent activity with IC50 values of approximately 0.1 μM, and weak cytotoxicity against mammalian cells. However, in vivo antimalarial activity is not effective which might be ascribed to the poor solubility of these compounds.
Conclusion: In this study, phenotypic screen of our compound library resulted in the first report of 3-cinnamamide framework with antimalarial activity and 40 derivatives were then designed and synthesized. Subsequent structure-activity studies showed that compounds 11, 23, 30 and 31 exhibited the most potent and selective activity against P. falciparum 3D7 strain with IC50 values around 0.1 μM. Our work herein sets another example of phenotypic screen-based drug discovery, leading to potentially promising candidates of novel antimalarial agents once given further optimization.
Graphical Abstract
Share this article
Medicinal Chemistry received 6627 citations as per Google Scholar report